ST. PAUL, Minn., Jan. 14, 2026 /PRNewswire/ -- iotaMotion, Inc., creator of iotaSOFT, the first and only FDA cleared robotic-assisted cochlear implant insertion system, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for expanded pediatric use of its iotaSOFT Insertion System. The system is now cleared for use in patients four years of age and older, extending access to robotic-assisted cochlear implantation for school-aged children.
"Receiving FDA clearance for expanded pediatric use marks a significant milestone for iotaMotion and for families navigating cochlear implant decisions," said Mike Lobinsky, CEO of iotaMotion. "By standardizing one of the most delicate steps in cochlear implant surgery, iotaSOFT helps reduce variability which could provide parents greater confidence as they consider cochlear implantation for their children."
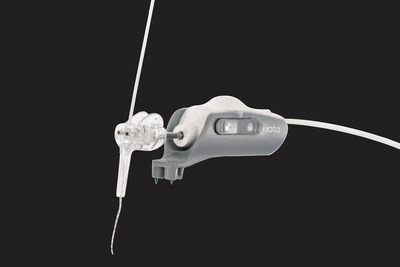
The iotaSOFT Insertion System enables precise and controlled electrode array insertion during one of the most delicate steps of cochlear implant surgery. By standardizing this critical step, iotaSOFT is designed to help preserve delicate cochlear structures, a consideration that is often central to decision-making.
As part of this milestone, Cincinnati Children's Hospital, a nationally ranked pediatric medical center, and U.S. News & World Report Honor Roll member for 2025-2026, has become the first dedicated pediatric center to adopt the iotaSOFT Insertion System. Cincinnati Children's joins over 35 leading cochlear implant centers across the United States, including nearly half of U.S. neurotology fellowship training programs, that have adopted this innovative technology.
"At Cincinnati Children's, our focus is delivering the highest standard of care while thoughtfully integrating innovations that benefit our patients," said Dr. Daniel Choo, chief clinical growth officer and professor of otolaryngology-head and neck surgery. "Robotic-assisted cochlear implantation represents a meaningful advancement in our cochlear implant program."
Globally, fewer than 5% of eligible cochlear implant candidates receive an implant, despite more than 430 million people worldwide experiencing disabling hearing loss, according to the World Health Organization. In pediatric patients, early access to sound is critical for speech, language and educational development, yet concerns around hearing preservation and surgical variability can delay intervention.
A 2025 clinical cohort study published in The Laryngoscope (Khan et al.) found that 85% of patients in the robotic-assisted insertion group maintained hearing preservation at one year, compared with 71% in the manual insertion group, according to its authors.
"Robotic assistance in cochlear implantation is about precision and consistency," said Marlan Hansen, MD, co-founder and chief medical officer of iotaMotion. "With the expanded pediatric indication for iotaSOFT, supported by growing clinical evidence, families can have greater confidence that controlled, standardized insertion is designed to protect the cochlea and preserve its structure and function. This is especially important for children who may benefit from emerging therapeutic advances, including gene-based and regenerative hearing technologies, which will likely depend on atraumatic, cochlear implant array placement early in life."
About iotaMotion
iotaMotion, Inc. is a medical technology company based in St. Paul, Minnesota, dedicated to advancing cochlear implant surgery beyond human capability through robotic-assisted solutions. Its flagship technology, the iotaSOFT Insertion System, is designed to preserve delicate intracochlear structures through slow and consistent electrode array insertion. The system is commercially available in the United States and under clinical investigation in other global markets.
For more information, visit http://www.iotamotion.com/ and follow us on LinkedIn, X, Facebook, YouTube.
Sources:
Claussen AD, et al. Comparative Analysis of Robotics-Assisted and Manual Insertions of Cochlear Implant Electrode Arrays. Otol Neurotol, 2022.
Khan AM, et al. Robotic-Assisted Electrode Array Insertion Improves Rates of Hearing Preservation. The Laryngoscope, 2025.
Data on file
Media Contact:
Sara Ashburn-Reed
Sashburn-reed@iotamotion.com
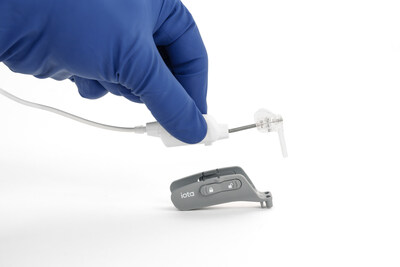

Photo - https://mma.prnewswire.com/media/2861279/iotaMotion_Robotic_Assisted_Cochlear_Implant_Insertion_System.jpg
Photo - https://mma.prnewswire.com/media/2861280/iotaSOFT_Robotic_Assisted_Insertion_System.jpg
Logo - https://mma.prnewswire.com/media/545464/IOTAMOTION_NEW.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/iotamotion-receives-fda-clearance-for-expanded-pediatric-use-of-iotasoft-robotic-assisted-cochlear-implant-insertion-system-302660704.html
View original content:https://www.prnewswire.co.uk/news-releases/iotamotion-receives-fda-clearance-for-expanded-pediatric-use-of-iotasoft-robotic-assisted-cochlear-implant-insertion-system-302660704.html

