Inclusion in new Acute Ischemic Stroke guidelines underscores clinical utility of Phagenyx for accelerating recovery from swallowing difficulties (dysphagia) in stroke patients
NASHVILLE, Tenn., Jan. 27, 2026 /PRNewswire/ -- Phagenesis, a commercial-stage company with a first-of-its-kind neuromodulation system to treat patients experiencing difficulty swallowing post-stroke, welcomed the 2026 Guideline for the Early Management of Patients With Acute Ischemic Stroke: A Guideline From the American Heart Association/American Stroke Association recognizing pharyngeal electrical stimulation (PES) for its role in restoring swallowing control in patients recovering from a stroke.
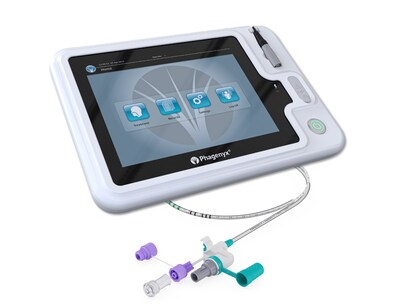
As the only commercially available solution for delivering PES to patients, the Phagenyx System applies electrical stimulation to the sensory nerves in the throat to restore the brain's control of swallowing function, accelerating recovery from severe dysphagia post-stroke. The new AHA/ASA clinical practice guidelines recommend that PES can be beneficial to reduce dysphagia severity, decrease the risk of aspiration and expedite decannulation.
"Dysphagia is among the most common and clinically significant complications following a stroke. It is a major contributor to reduced airway protection, leading to aspiration pneumonia and the need for tracheostomy. Beyond its medical consequences, dysphagia deprives patients from the basic pleasure and dignity of eating and drinking," stated H. Alex Choi, MD, MS, Professor of Neurosurgery and Neurology at The University of Texas Health Science Center at Houston. "Yet despite the prevalence and impact of dysphagia, we have historically had few meaningful therapeutic interventions to improve outcomes for patients."
Dysphagia impacts 45% of stroke patients1 and is associated with serious complications such as a 3-times higher risk of pneumonia2 and a 7-times higher risk of needing a permanent feeding tube3 as well as a higher risk of hospital readmission. Additionally, patients with dysphagia have an 8-times higher risk of death at 3 months.4
The AHA/ASA stroke guideline includes the following recommendations for dysphagia:
- In patients with stroke with dysphasia, treatment with PES, can be beneficial to reduce dysphagia severity and decrease the risk of aspiration. Class of Recommendation 2a, Level of Evidence B-R
- In patients with severe stroke with dysphagia requiring tracheotomy and mechanical ventilation, treatment with PES, after ventilator weaning can be beneficial to decrease dysphagia severity, reduce the risk of aspiration and expedite decannulation. Class of Recommendation 2a, Level of Evidence B-R
"The addition of PES to the guidelines signals that we now have a validated therapeutic option that can be integrated into routine stroke care, giving clinicians a tool to reduce complications, help patients eat and drink again, improving the quality of life for our patients. Dysphagia should no longer be viewed simply as a barrier to discharge from the hospital but rather a therapeutic target," added Dr. Choi.
Phagenyx is supported by more than 30 peer-reviewed publications, including 9 randomized controlled trials and 13 mechanistic studies. These studies demonstrate that PES with Phagenyx provides a 2-times improvement in swallowing safety5 and a significant reduction in dysphagia severity scores (FEDSS),6 supporting safer oral intake and reduced reliance on feeding tubes.
"The addition of PES to the stroke care guideline is a powerful validation of the work our clinical partners and team have undertaken over many years," said Chad Hoskins, Chief Executive Officer of Phagenesis. "The formal inclusion reinforces the value of Phagenyx in improving outcomes for stroke survivors, while also reducing cost and complexity for healthcare providers. We appreciate the AHA's recognition of dysphagia as a barrier to recovery and PES as a tool to address it."
ABOUT PHAGENESIS
Phagenesis is a leading medical technology company transforming the treatment of neurogenic dysphagia with targeted pharyngeal electrical stimulation (PES). Its Phagenyx System is a clinically validated neuromodulation therapy that helps restore safe, efficient swallowing in patients impacted by stroke or other neurological injury. In the United States, Phagenyx is indicated for severe post-stroke dysphagia, and in Europe it is CE-marked for neurogenic dysphagia caused by a range of neurological conditions. The company is backed by EQT Life Sciences, Sectoral Asset Management, British Patient Capital, Northern Gritstone and Aphelion/Cardeation. For additional information, visit www.phagenesis.com.
References:
- Song W, et al; Frontiers Neurol, 2024;1, 1403610.
- Martino, R., et al. (2005). Stroke, 36(12), 2756-2763
- Vasan, V., et al. (2023). Journal of Stroke and Cerebrovascular Diseases, 32(9), 107295.
- Arnold, M., et al (2016). PloS one, 11(2), e0148424.
- Youssef G, El-Banna M. Al-Azhar Assiut Med Journal. 2015; 13(1):68-72.
- Suntrup-Krueger et al. Neurotherapeutics. 2025

Photo - https://mma.prnewswire.com/media/2870649/Phagenesis__Phagenyx____A_clinically_validated_neuromodulation_system_using_pharyngeal_electrical.jpg
Logo - https://mma.prnewswire.com/media/2870648/PHAGENESIS__Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/pharyngeal-electrical-stimulation-recognized-as-important-tool-for-post-stroke-recovery-in-new-american-heart-association-and-american-stroke-association-guideline-302671505.html
View original content:https://www.prnewswire.co.uk/news-releases/pharyngeal-electrical-stimulation-recognized-as-important-tool-for-post-stroke-recovery-in-new-american-heart-association-and-american-stroke-association-guideline-302671505.html

