Safety concern warrants reevaluation of study design, including enrollment criteria
Potential future trial with Auxora in AKI to be evaluated after data analysis
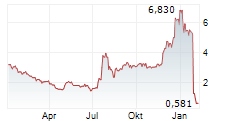
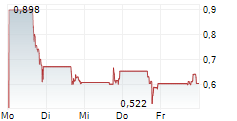
LA JOLLA, Calif., Jan. 28, 2026 /PRNewswire/ -- CalciMedica Inc. ("CalciMedica" or the "Company") (Nasdaq: CALC), a clinical-stage biopharmaceutical company developing novel calcium release-activated calcium (CRAC) channel inhibition therapies for acute and chronic inflammatory and immunologic illnesses, today announced the discontinuation of its Phase 2 KOURAGE clinical trial evaluating Auxora in patients with Stage 2 or Stage 3 acute kidney injury (AKI) with associated acute hypoxemic respiratory failure (AHRF), a population characterized by high mortality and a lack of approved therapies. The decision follows a recommendation from the trial's Independent Data Monitoring Committee (IDMC).
During a prescheduled interim data review, the IDMC identified a safety concern that, in its view, warrants reevaluation of the study design, particularly with respect to patient enrollment criteria. There were no deaths in the trial that were assessed by investigators or CalciMedica as being related to study drug (either Auxora or placebo). In addition, no serious adverse events met the criteria for expedited reporting to the U.S. Food and Drug Administration (FDA). Based on the IDMC's recommendation, the Company has discontinued the trial and plans to perform a comprehensive review of the unblinded clinical data.
The Company will assess the impact of baseline characteristics, disease severity, concomitant therapies, and other factors on patient outcomes. These analyses are expected to inform how future clinical evaluation of Auxora in AKI may proceed.
"Based on the IDMC's feedback, we will review the unblinded KOURAGE data and explore modifications to the trial design, particularly patient enrollment criteria, that may support further clinical testing of Auxora in patients with AKI," said Rachel Leheny, Ph.D., CEO of CalciMedica. "To date, no treatment-related safety concern has been observed across more than 350 critically ill patients treated with Auxora in completed clinical trials, including the Phase 2b CARPO trial in acute pancreatitis (AP) and the Phase 2 CARDEA trial in severe COVID-19 pneumonia. We remain optimistic about Auxora's potential in acute inflammatory illnesses and look forward to finalizing the design of a pivotal trial in AP, pending FDA feedback, in the first half of 2026, while also advancing a second CRAC channel inhibitor, CM5480, as a potential first-in-class, differentiated therapy for pulmonary arterial hypertension."
The Company has notified the FDA about the trial discontinuation and will work with investigators to ensure all patients currently enrolled in the trial complete the full 90-day follow-up.
About CalciMedica
CalciMedica is a clinical-stage biopharmaceutical company developing novel calcium release-activated calcium (CRAC) channel inhibition therapies for inflammatory and immunologic diseases. CalciMedica's proprietary technology targets the inhibition of CRAC channels to modulate the immune response and protect against tissue cell injury, with the potential to provide therapeutic benefits in in serious and life-threatening conditions with high unmet need. CalciMedica's lead product candidate, Auxora, has demonstrated positive clinical results in multiple completed efficacy clinical trials. The Company has reported data from a Phase 2b trial (CARPO; NCT04681066) evaluating Auxora in patients with acute pancreatitis (AP) and accompanying systemic inflammatory response syndrome (SIRS), as well as from a Phase 2 trial (CARDEA; NCT04345614) in patients with severe COVID-19 pneumonia. In addition, CalciMedica is advancing CM5480 as a potential therapy for pulmonary arterial hypertension (PAH), supported by preclinical data demonstrating effects on pulmonary vascular remodeling and right ventricular function. For more information, please visit www.calcimedica.com.
Forward-Looking Statements
This communication contains forward-looking statements which include, but are not limited to CalciMedica's planned and ongoing clinical trials and the timing, design, expected patient enrollment thereof and the expected timing for updates; statements regarding the safety and efficacy of its product candidates; statements regarding the planned analysis of the unblinded KOURAGE dataset, including the timing of such analysis and whether such analysis will inform future trial parameters; and the potential of CalciMedica's proprietary technology to provide therapeutic benefits in PAH and other acute and chronic inflammatory and immunologic diseases such as AKI and AP. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. CalciMedica's expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties related to: the impact of fluctuations in global financial markets on CalciMedica's business and the actions it may take in response thereto; CalciMedica's ability to execute its plans and strategies; the ability to obtain and maintain regulatory approval for Auxora; results from clinical trials or preclinical studies may not be indicative of results that may be observed in the future; potential safety and other complications from Auxora; the scope, progress and expansion of developing and commercializing Auxora; the size and growth of the market therefor and the rate and degree of market acceptance thereof; economic, business, competitive, and/or regulatory factors affecting the business of CalciMedica generally; CalciMedica's ability to protect its intellectual property position; the impact of government laws and regulations; and CalciMedica's financial position and need for additional capital. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption "Risk Factors" in CalciMedica's Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, filed with the Securities and Exchange Commission (SEC) on November 12, 2025, and elsewhere in CalciMedica's subsequent reports on Form 10-K, Form 10-Q or Form 8-K filed with the SEC from time to time and available at www.sec.gov. These documents can be accessed on CalciMedica's web page at ir.calcimedica.com/financials-filings/sec-filings. The forward-looking statements contained herein are made as of the date hereof, and CalciMedica undertakes no obligation to update them after this date, except as required by law.
Contact Information
Kevin Murphy
[email protected]
(212) 600-1902
SOURCE CalciMedica, Inc.