Sandoz Group AG
/ Key word(s): Regulatory Approval
MEDIA RELEASE
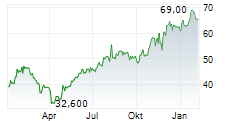
Basel, February 18, 2026 - Sandoz (SIX:SDZ/OTCQX:SDZNY), the global leader in affordable medicines, today announced that the US Food and Drug Administration (FDA) has approved an expanded label for Enzeevu (aflibercept-abzv), to include multiple retinal indications. Enzeevu was originally approved by the FDA for the treatment of neovascular (wet) age-related macular degeneration (nAMD) in August 20242.
End of Media Release |
| Language: | English |
| Company: | Sandoz Group AG |
| Centralbahnstrasse 4 | |
| 4051 Basel | |
| Switzerland | |
| Internet: | www.sandoz.com |
| ISIN: | CH1243598427 |
| Valor: | 124359842 |
| Listed: | SIX Swiss Exchange |
| EQS News ID: | 2277560 |
| End of News | EQS News Service |
2277560 18.02.2026 CET/CEST