- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 19.12.25 | APTAHEM: Newsletter December 2025 | 1 | Cision News | ||
| 05.12.25 | Aptahem receives patent protection in China for patent family 2 | 2 | Cision News | ||
| APTAHEM Aktie jetzt für 0€ handeln | |||||
| 30.10.25 | Aptahem's webinar "Strategic Direction and Planned US Listing" now available | 2 | Cision News | ||
| 22.10.25 | Aptahem Announces English-Language Webinar on Strategic Direction and Planned U.S. Listing | 2 | Cision News | ||
| 21.10.25 | APTAHEM: Newsletter October 2025 | 1 | Cision News | ||
| 03.10.25 | Spotlight Stock Market: Market Notice 186/25 - Last day of trading in Aptahem AB's BTA | 1 | Cision News | ||
| 25.09.25 | Aptahem moves forward with focus on U.S. listing strategy, regulatory progress, and partner engagement following outcome of rights issue | 1 | Cision News | ||
| 23.09.25 | Aptahem receives Notice of Allowance in China for patent family 2 | 2 | Cision News | ||
| 19.09.25 | Aptahem Outlines Ongoing Preparations for Planned U.S. Listing and Announces Webinar Invitation | 1 | Cision News | ||
| 02.09.25 | Spotlight Stock Market: Market Notice 164/25 - Information regarding the rights issue from Aptahem AB | 1 | Cision News |
10 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BIONTECH | 93,10 | -0,21 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report | ||
| PRAXIS PRECISION MEDICINES | 336,75 | -1,05 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,820 | -3,48 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| RELAY THERAPEUTICS | 10,250 | +12,02 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| ADMA BIOLOGICS | 15,580 | +2,70 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| EDGEWISE THERAPEUTICS | 30,450 | +2,77 % | Edgewise Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results with Strong Progress Across Muscular Dystrophy and Cardiovascular Programs | - CIRRUS-HCM 12-week data of EDG-7500 in obstructive and nonobstructive hypertrophic cardiomyopathy (HCM) expected in H1 2026 -
- Phase 1 healthy adult trial... ► Artikel lesen | |
| COGENT BIOSCIENCES | 38,870 | -1,55 % | Analyst Bullish on Cogent (COGT) Amid Expected Drug Approval | ||
| PRIME MEDICINE | 4,630 | +5,71 % | Prime Medicine, Inc.: Prime Medicine Reports Third Quarter 2025 Financial Results and Provides Business Updates | -- New preclinical data for PM577 in Wilson's Disease (WD) to be presented at AASLD; on-track to file IND and/or CTA in H1'26, with initial clinical data expected in 2027 -- -- Nominated PM647 as... ► Artikel lesen |
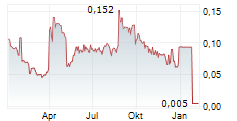
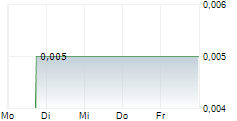
APTAHEM AB gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 0,005 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu APTAHEM AB finden Sie auf Wallstreet Online.