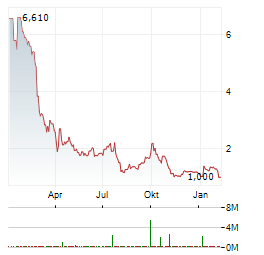
Chart-Typ:
Zeitraum:
| Weekly Buzz: ARS Pharma's Neffy Goes East, SLS Advances, A Signal Of Hope For IFRX? | LONDON (dpa-AFX) - Despite the typical year-end slowdown, the week saw a steady stream of high-impact news, highlighted by global approvals, clinical trial readouts, new product launches, and... ► Artikel lesen |
| InflaRx N.V.: InflaRx Provides Update on Phase 3 Data Analyses for Vilobelimab in Pyoderma Gangrenosum | While the Phase 3 trial of vilobelimab in ulcerative pyoderma gangrenosum (PG) was terminated earlier this year due to futility regarding its prespecified primary endpoint (as previously disclosed)... ► Artikel lesen |
| InflaRx N.V.: InflaRx Announces International Nonproprietary Name of "Izicopan" for INF904 | JENA, Germany, Dec. 11, 2025 (GLOBE NEWSWIRE) -- InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical company pioneering anti-inflammatory therapeutics by targeting the complement system, today announced... ► Artikel lesen |
| XFRA 7300,B0Y,YAG: AUSSETZUNG/SUSPENSION | DAS/ DIE FOLGENDE(N) INSTRUMENT(E) IST/ SIND AB SOFORT AUSGESETZT:THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTILZEROSTACK CORP.... ► Artikel lesen |
| XFRA NEW INSTRUMENTS AVAILABLE ON 02.02.2026 | The following instruments on XETRA do have their first trading 02.02.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 02.02.2026
Aktien
1 CA00249P1053 AXA S.A. CDR
2 ARP125991090... ► Artikel lesen |
| XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 30.01.2026 | he following instruments on Boerse Frankfurt do have their last trading day on 30.01.2026.Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 30.01.2026.ISIN NameCA3397643006 FLORA... ► Artikel lesen |
| RedHill Biopharma Ltd.: RedHill Biopharma's Positive Opaganib Results Indicate Reduction in Venetoclax Resistant Cells | Resistance to venetoclax (Venclexta® and Venclyxto®, Abbvie / Genentech), the $2.5 billion blockbuster Chronic Lymphocytic Leukemia (CLL) therapy, is emerging as... ► Artikel lesen |
| RedHill Biopharma Ltd.: RedHill Biopharma Successfully Regains Compliance with Nasdaq Stockholders' Equity Requirement | TEL-AVIV, Israel and RALEIGH, N.C., Dec. 1, 2025 /PRNewswire/ -- RedHill Biopharma Ltd. (NASDAQ: RDHL) ("RedHill" or the "Company"), a specialty biopharmaceutical... ► Artikel lesen |
| RedHill Biopharma Receives Nasdaq Staff Determination Notification | On October 16, 2025, the Company received a Staff Determination notification from The Nasdaq Stock Market LLC regarding continued non-compliance with the minimum... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GENELUX CORPORATION finden Sie auf Wallstreet Online.