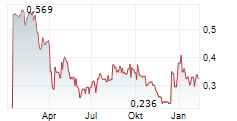
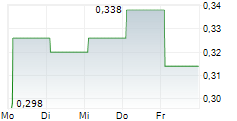
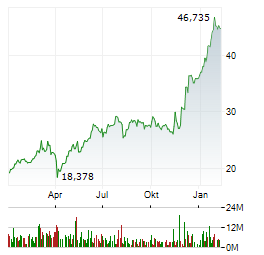
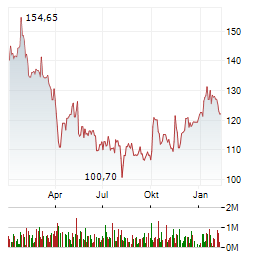
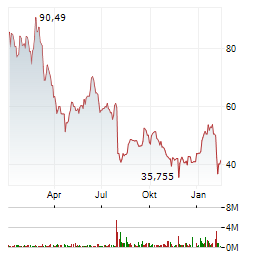
Chart-Typ:
Zeitraum:
| BASF, K+S, Bayer: Iran-Konflikt spaltet Chemiebranche: Das sind die Gewinner und Verlierer | © Foto: dpa-BildfunkWährend Rüstungswerte wie Rheinmetall zulegen, stehen viele Chemietitel unter Druck. Investoren fürchten vor allem eines: eine neue Energiepreis-Welle mit globalen Folgen. Doch es... ► Artikel lesen |
| Rheinmetall, Infineon, Bayer & Co. sind es nicht! ... und wie Börsenweisheiten über das schwierige Jahr 2025 hinweghelfen konnten. | Guten Tag, liebe Leserinnen und Leser,
viele von Ihnen kennen sie wahrscheinlich gar nicht mal. Aber vielleicht ist Ihnen doch schon die ein oder andere Börsenweisheit untergekommen, die aufgrund ihrer... ► Artikel lesen |
| Die Lösung für den Hunger einer wachsenden Weltbevölkerung: Bayer, K+S und MustGrow Biologics! Senf als biologische Innovationen kann die Landwirtschaft von morgen verändern! | Die Menschheit hat ein großes Problem, das alle Menschen betrifft. Auf der Erde leben heute mehr als 8 Milliarden Menschen. Jeder Mensch braucht jeden Tag Essen. Aber die Felder, auf denen wir Lebensmittel... ► Artikel lesen |
| Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | Anzeige / WerbungBig Blue IBM, Fastly & Merck - Was sagen Charts UND Fundamentaldaten wirklich? Fundamentalanalyse trifft auf Charttechnik: Im zweiten Webinar der Reihe 2026 nehmen Michael und Andreas... ► Artikel lesen |
| EQS-PVR: Merck KGaA: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: Merck KGaA
Merck KGaA: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide... ► Artikel lesen |
| ROUNDUP/Chefwechsel bei Sanofi: Scheidende Merck-Lenkerin kommt | PARIS/DARMSTADT (dpa-AFX) - Der Pharmakonzern Sanofi löst überraschend seinen bisherigen Chef Paul Hudson ab. Der Manager wird das Unternehmen bereits am 17. Februar verlassen, wie der französische... ► Artikel lesen |
| Election of employee representatives to the Board of Directors of Novo Nordisk A/S | Bagsværd, Denmark, 2 March 2026 - The employees in Novo Nordisk A/S have completed the election of employee representatives to the Board of Directors of Novo Nordisk A/S.
The following employee... ► Artikel lesen |
| Novo Nordisk: Analyst senkt Kursziel um 35 Prozent - dieser Support muss jetzt halten | Kräftig im Minus ist die Aktie von Novo Nordisk heute in den Handel gestartet. Neben der allgemeinen Marktschwäche sorgte insbesondere eine Abstufung aus den USA für ordentlich Druck. Positiv wurde... ► Artikel lesen |
| Novo Nordisk investiert 432 Millionen Euro für Produktion von Abnehm-Medikamenten | Der dänische Pharmakonzern Novo Nordisk baut seine Produktionskapazitäten für orale GLP-1-Präparate mit einer Investition von 432 Millionen Euro in seinem irischen Werk in Athlone aus. Die Mittel fließen... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GROWN ROGUE INTERNATIONAL INC finden Sie auf Wallstreet Online.