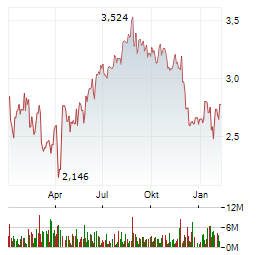
Chart-Typ:
Zeitraum:
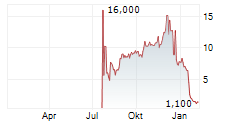
| EQS-NVR: Vonovia SE: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG mit dem Ziel der europaweiten Verbreitung | EQS Gesamtstimmrechtsmitteilung: Vonovia SE
/ Veröffentlichung der Gesamtzahl der Stimmrechte
Vonovia SE: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG mit dem... ► Artikel lesen |
| Dax will die 25.000 - Alzchem, Auto1, E.on, Fresenius, Heidelberg Materials, Nordex, Vonovia ... | Der DAX nimmt die 25.000-Punkte-Marke erneut ins Visier - Rückenwind kommt von stabilen US-Vorgaben und der Hoffnung auf starke Zahlen von Nvidia. Trotz neuer Zoll-Debatten aus Washington bleibt die... ► Artikel lesen |
| DAX-Check LIVE: Alzchem, Auto1, E.on, Fresenius, Heidelberg Materials, Nordex, Vonovia im Fokus | Der DAX startete gestern deutlich schwächer, konnte die Verluste im Tagesverlauf jedoch weitgehend aufholen und schloss 0,02 Prozent höher bei 24.986 Punkten. Belastend wirkten weiterhin Unsicherheiten... ► Artikel lesen |
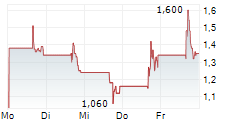
| EQS-PVR: LEG Immobilien SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: LEG Immobilien SE
LEG Immobilien SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective... ► Artikel lesen |
| EQS-PVR: LEG Immobilien SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective of Europe-wide distribution | EQS Voting Rights Announcement: LEG Immobilien SE
LEG Immobilien SE: Release according to Article 40, Section 1 of the WpHG [the German Securities Trading Act] with the objective... ► Artikel lesen |
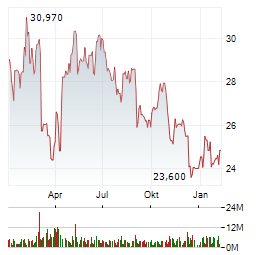
| Vonovia, LEG Immobilien und Co: Das sieht immer besser aus | Vor gut einer Woche hat DER AKTIONÄR darauf hingewiesen, dass sich bei den Aktien deutscher Wohnimmobilienunternehmen nach mehrmonatiger Talfahrt eine Trendwende andeutet. Ein Blick auf die Charts der... ► Artikel lesen |
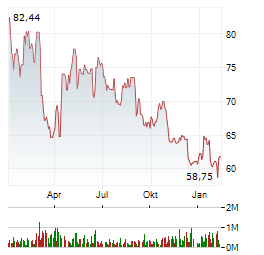
| EQS-CMS: Aroundtown SA: Release of a capital market information | EQS Post-admission Duties announcement: Aroundtown SA
/ Disclosure pursuant to Art. 5 Sec. 1 lit. b) and Sec. 3 of the Regulation (EU) No 596/2014 (MAR) in conjunction with Art. 2... ► Artikel lesen |
| EQS-CMS: Aroundtown SA: Release of a capital market information | EQS Post-admission Duties announcement: Aroundtown SA
/ Disclosure pursuant to Art. 5 Sec. 1 lit. b) and Sec. 3 of the Regulation (EU) No 596/2014 (MAR) in conjunction with Art. 2... ► Artikel lesen |
| BARCLAYS stuft Aroundtown auf 'Underweight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat Aroundtown auf "Underweight" mit einem Kursziel von 2,10 Euro belassen. Zuletzt sei der Gewerbeimmobilien-Spezialist Thema zweier... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LINKHOME HOLDINGS INC finden Sie auf Wallstreet Online.