Chart-Typ:
Zeitraum:
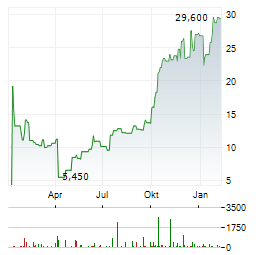
| Oruka Therapeutics, Inc.: Oruka Therapeutics Reports Third Quarter 2025 Financial Results and Provides Corporate Update | ORKA-001 Phase 1 results presented at EADV show potential for once-per-year dosing, higher efficacy and off-treatment remission Over $500M cash and equivalents provides runway over one year past three... ► Artikel lesen |
| Oruka Therapeutics, Inc.: Oruka Therapeutics Announces Positive Interim Phase 1 Results for ORKA-001 | Half-life of approximately 100 days increases likelihood of once-per-year dosing Pharmacokinetic profile supports the ability to achieve exposures that could lead to higher efficacy and extended... ► Artikel lesen |
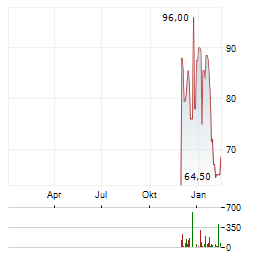
| Palvella Therapeutics Inc.: Palvella Therapeutics Announces Closing of Upsized Public Offering of Common Stock and Exercise in Full of the Underwriters' Option to Purchase Additional Shares | WAYNE, Pa., March 02, 2026 (GLOBE NEWSWIRE) -- Palvella Therapeutics, Inc. ("Palvella") (Nasdaq: PVLA), a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapies... ► Artikel lesen |
| Palvella Therapeutics Inc.: Palvella Therapeutics Announces Positive Topline Results from Phase 3 SELVA Clinical Study of QTORIN 3.9% Rapamycin Anhydrous Gel (QTORIN rapamycin) in Microcystic Lymphatic Malformations | Primary endpoint met with statistically significant improvement (mean change of +2.13; p Achieved statistical significance on pre-specified key secondary endpoint (p 95% of trial participants aged... ► Artikel lesen |
| Palvella Therapeutics Inc.: Palvella Therapeutics Provides Corporate Update and 2026 Outlook: Advancing a Late Clinical-Stage Pipeline and Platform to Address Multiple Serious, Rare Skin Diseases and Vascular Malformations with No FDA-Approved ... | Phase 3 SELVA study evaluating QTORIN rapamycin 3.9% anhydrous gel (QTORIN rapamycin) for microcystic lymphatic malformations (microcystic LMs) remains on track, with topline results anticipated in... ► Artikel lesen |
| Morning Market Movers: Neurogene, Duolingo, Xponential Fitness, Emergent BioSolutions See Big Swings | WASHINGTON (dpa-AFX) - At 6:22 a.m. ET on Friday, premarket trading is seeing notable activity in several stocks, with early price movements signaling potential opportunities before the opening... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
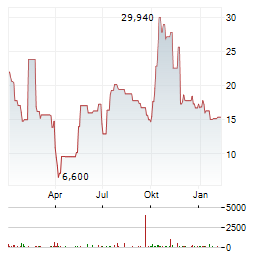
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PARDES BIOSCIENCES INC finden Sie auf Wallstreet Online.