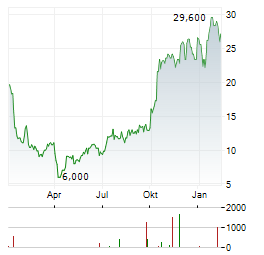
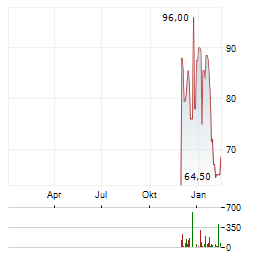
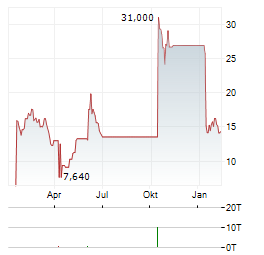
Chart-Typ:
Zeitraum:
| Oruka Therapeutics, Inc.: Oruka Therapeutics Reports Third Quarter 2025 Financial Results and Provides Corporate Update | ORKA-001 Phase 1 results presented at EADV show potential for once-per-year dosing, higher efficacy and off-treatment remission Over $500M cash and equivalents provides runway over one year past three... ► Artikel lesen |
| Oruka Therapeutics, Inc.: Oruka Therapeutics Announces Positive Interim Phase 1 Results for ORKA-001 | Half-life of approximately 100 days increases likelihood of once-per-year dosing Pharmacokinetic profile supports the ability to achieve exposures that could lead to higher efficacy and extended... ► Artikel lesen |
| Oruka Therapeutics, Inc.: Oruka Therapeutics Reports Second Quarter 2025 Financial Results and Provides Corporate Update | First patients dosed in the EVERLAST-A Phase 2a trial of ORKA-001, with data expected in 2H 2026 ORKA-001 Phase 1 data and EVERLAST-A design to be presented at EADV in September 2025 ORKA-002 Phase... ► Artikel lesen |
| Palvella Therapeutics Inc.: Palvella Therapeutics Provides Corporate Update and 2026 Outlook: Advancing a Late Clinical-Stage Pipeline and Platform to Address Multiple Serious, Rare Skin Diseases and Vascular Malformations with No FDA-Approved ... | Phase 3 SELVA study evaluating QTORIN rapamycin 3.9% anhydrous gel (QTORIN rapamycin) for microcystic lymphatic malformations (microcystic LMs) remains on track, with topline results anticipated in... ► Artikel lesen |
| Palvella Therapeutics Inc.: Palvella Therapeutics Strengthens Leadership Team with Appointment of Veteran Medical Affairs Leader Vimal Patel, PharmD, as Senior Vice President of Medical Affairs | Seasoned dermatology and immunology executive brings more than 25 years of experience advancing and launching innovative therapies, including OPZELURA- , povorcitinib, ILUMYA®, ODOMZO- , REMICADE-... ► Artikel lesen |
| Palvella Therapeutics Inc.: Palvella Therapeutics Granted FDA Fast Track Designation for QTORIN 3.9% Rapamycin Anhydrous Gel (QTORIN rapamycin) for the Treatment of Angiokeratomas | Fast Track designation designed to facilitate the development, and expedite the review, of drugs to treat serious conditions and fill an unmet need With Fast Track designation, QTORIN rapamycin for... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu THESEUS PHARMACEUTICALS INC finden Sie auf Wallstreet Online.