SUZHOU, China, Jan. 10, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited (HKEX: 9939) is pleased to update the preliminary analysis of the clinical trial of Proxalutamide for the treatment of COVID-19 patients. The investigator initiated trial conducted by Dr. Andy Goren and Dr. Flávio Adsuara Cadegiani, shows promising results in the treatment of COVID-19 female outpatients.
This trial commenced the enrolment of 168 female patients on Nov, 2020 after receiving the encouraging results from male patients. Eligible female patients were randomized in a 2:1 ratio to receive either Proxalutamide + standard care (Proxalutamide arm) or Placebo + standard care (Control arm). The co-primary endpoints of the clinical trial are the percentage of subjects hospitalized with COVID-19 and the COVID-19 Ordinal Outcome Scale (a 8-point ordinal scale used by the US National Institute of Allergy and Infection Diseases, such as mechanical ventilation usage and death) in 30 days.
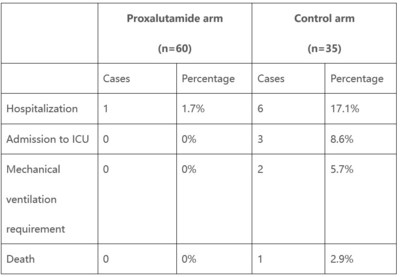
The interim analysis conducted on Jan 7, 2021 was based on 60 patients in Proxalutamide arm and 35 patients in the control arm. The percentage of hospitalization, admission to ICU, mechanical ventilation requirement and death was 1.7% vs. 17.1%, 0% vs. 8.6%, 0% vs. 5.7% and 0%, vs. 2.9% in Proxalutamide and placebo arms respectively.
The trial is expected to conclude enrolment in February 2021, and finalize the data collection by March 2021.
Dr. Tong Youzhi, the founder, Chairman and CEO of Kintor Pharmaceutical, said, "first of all, we would like to give special thanks to all the healthcare workers fighting the worldwide COVID-19 pandemic. Special thanks to the AndroCoV[1][2] group led by Dr. Andy Goren, Dr. Flávio Adsuara Cadegiani, Dr. John McCoy and Dr. Carlos Gustavo Wambier, who are conducting this important investigator initiated study. Although the female group has lower androgen level as compared to male group, the data showed that Proxalutamide could significantly ameliorate symptoms and prevent hospitalization for COVID-19 female patients, which paves the way for our phase III MRCT registrational trial in the near future."
Related Links
[1]Ministry of Health prepares actions to strengthen the SUS in Manaus
https://www.gov.br/saude/pt-br/assuntos/noticias/ministerio-da-saude-prepara-acoes-para-reforco-do-sus-em-manaus
[2]COVID-19 AndroCoV Protocol Pioneered by Applied Biology and Brazilian Scientists Adopted by the Brazilian Government
https://www.einnews.com/pr_news/534176202/covid-19-androcov-protocol-pioneered-by-applied-biology-and-brazilian-scientists-adopted-by-the-brazilian-government
Photo - https://mma.prnewswire.com/media/1417332/image_Infographic.jpg

