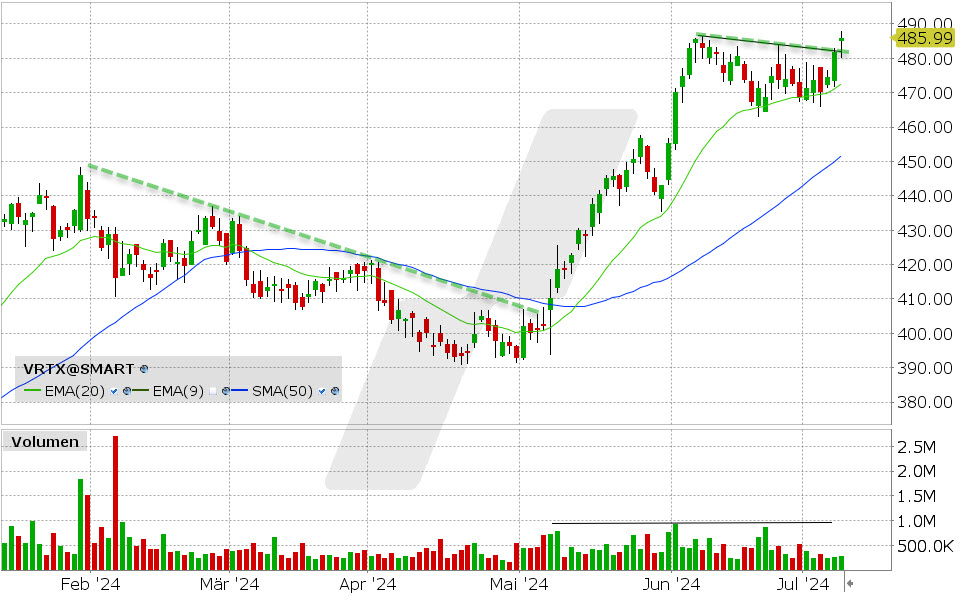
 Der Biotechnologiekonzern lieferte im gestrigen Handel erneut ein starkes Kaufsignal. Die Aktie von Vertex Pharmaceuticals befindet sich bereits seit Mai im Höhenflug und konnte seither +20% an Wert zulegen.Den vollständigen Artikel lesen ...
Der Biotechnologiekonzern lieferte im gestrigen Handel erneut ein starkes Kaufsignal. Die Aktie von Vertex Pharmaceuticals befindet sich bereits seit Mai im Höhenflug und konnte seither +20% an Wert zulegen.Den vollständigen Artikel lesen ...© 2024 LYNX-Werbemitteilung - Bitte beachten Sie den Haftungsausschluss
