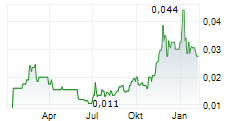
Actinogen Medical continues to make progress with its 36-week XanaMIA Phase IIb/III study assessing Xanamem in patients with biomarker-positive mild-to-moderate Alzheimer's disease (AD). The company recently announced it has randomised and treated 40 patients (out of the 220 target). Actinogen now expects to report interim (24-week) results from the first 100 patients in Q4 CY25 (vs Q3 CY25 previously), which could be a material catalyst and support licensing and/or value realisation opportunities. Full study results are guided for H2 CY26. Our risk-adjusted net present value is A$673.8m (vs A$619.8m previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research