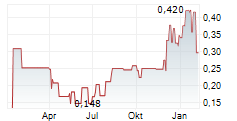
Recce has reported positive results from its open-label Phase II study assessing RECCE® 327 topical gel (R327G) in patients with acute bacterial skin and skin structure infections (ABSSSI), including patients with diabetic foot infections (DFIs). In 29 evaluable patients, the company reported a 93% primary efficacy endpoint with R327G treatment over 14 days. In our view, these results bode well for the company's Phase III registrational study in Indonesia assessing R327G as a treatment for DFIs. We expect patient dosing and recruitment to start imminently given the late CY24 approval for the study to commence from the Indonesian Drug and Food Regulatory Authority, Badan POM. If the results are positive, Recce could potentially launch R327G in South-East Asia in H2 CY26, marking the company's transition to commercial stage.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research