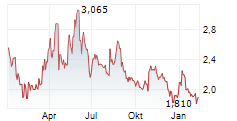
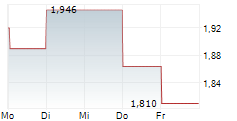
TURKU, FI / ACCESS Newswire / April 17, 2025 / Faron Pharmaceuticals Ltd. (AIM:FARN)(First North:FARON)(HEL:FARON)(LSE:FARN), a clinical-stage biopharmaceutical company focused on developing novel immunotherapies, announces that, on 16 April 2025, A&B (HK) Company Ltd (" A&B ") divested its entire shareholding of 3,559,893 ordinary shares representing approximately 3.40% of the Company's issued share capital.
A&B (HK) Company Ltd, an investment and development company based in Hong Kong, initially invested in the Company in 2015. This investment was part of a strategic agreement involving Faron's lead drug candidate, Traumakine®, which was developed for the treatment of moderate to severe acute respiratory distress syndrome (ARDS). As Faron's focus is on the development of its lead asset, bexmarilimab, this strategic investment by A&B no longer serves its original purpose and as such, A&B sold its holding and created a significant increase in the liquidity of Faron's shares in the market.
For more information please contact:
IR Partners, Finland (media)
Riina Tuominen
+358 44 313 5005
riina.tuominen@irpartners.fi
Kare Laukkanen
+358 50 553 9535 / +44 7 469 766 223
kare.laukkanen@irpartners.fi
FINN Partners, US (media)
Alyssa Paldo
+1 847 791-8085
alyssa.paldo@finnpartners.com
Cairn Financial Advisers LLP, Nominated Adviser and Broker
Sandy Jamieson, Jo Turner
Phone: +44 (0) 207 213 0880
Sisu Partners Oy, Certified Adviser on Nasdaq First North
Juha Karttunen
Phone: +358 (0)40 555 4727
Jukka Järvelä
Phone: +358 (0)50 553 8990
About BEXMAB
The BEXMAB study is an open-label Phase I/II clinical trial investigating bexmarilimab in combination with standard of care (SoC) in the aggressive hematological malignancies of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). The primary objective is to determine the safety and tolerability of bexmarilimab in combination with SoC (azacitidine) treatment. Directly targeting Clever-1 could limit the replication capacity of cancer cells, increase antigen presentation, ignite an immune response, and allow current treatments to be more effective. Clever-1 is highly expressed in both AML and MDS and associated with therapy resistance, limited T cell activation and poor outcomes.
About bexmarilimab
Bexmarilimab is Faron's wholly owned, investigational immunotherapy designed to overcome resistance to existing treatments and optimize clinical outcomes, by targeting myeloid cell function and igniting the immune system. Bexmarilimab binds to Clever-1, an immunosuppressive receptor found on macrophages leading to tumor growth and metastases (i.e. helps cancer evade the immune system). By targeting the Clever-1 receptor on macrophages, bexmarilimab alters the tumor microenvironment, reprogramming macrophages from an immunosuppressive (M2) state to an immunostimulatory (M1) one, upregulating interferon production and priming the immune system to attack tumors and sensitizing cancer cells to standard of care.
About Faron Pharmaceuticals Ltd
Faron (AIM:FARN, First North:FARON) is a global, clinical-stage biopharmaceutical company, focused on tackling cancers via novel immunotherapies. Its mission is to bring the promise of immunotherapy to a broader population by uncovering novel ways to control and harness the power of the immune system. The Company's lead asset is bexmarilimab , a novel anti-Clever-1 humanized antibody, with the potential to remove immunosuppression of cancers through reprogramming myeloid cell function. Bexmarilimab is being investigated in Phase I/II clinical trials as a potential therapy for patients with hematological cancers in combination with other standard treatments. Further information is available at www.faron.com.
SOURCE: Faron Pharmaceuticals