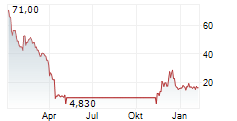
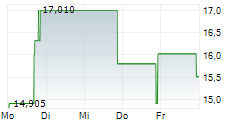
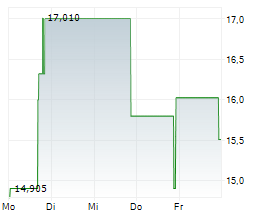
WASHINGTON (dpa-AFX) - Palatin Technologies, Inc. (PTN), a biopharmaceutical company, Tuesday reported updated results from responder analyses of its pivotal Phase 3 MELODY-1 study evaluating the safety and efficacy of PL9643 versus placebo in the treatment of dry eye disease (DED).
Responder analysis was conducted to evaluate the percentage of patients achieving complete symptom clearing (resolution) across 13 pre-specified symptom endpoints. In 6 of the 13 symptom endpoints, a significantly higher percentage of patients treated with PL9643 achieved complete symptom resolution compared to placebo at 12 weeks. Early and sustained symptom resolution was also observed in patients treated with PL9643.
'These results reinforce PL9643's potential as a best-in-class therapy with a differentiated mechanism of action. Combined with its rapid, sustained efficacy and excellent safety and tolerability profile, PL9643 offers a compelling new option for patients. Critically, these outcomes align with key FDA approval criteria for symptom improvement based on responder analyses,' said Carl Spana, President and CEO of Palatin Technologies.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News