WASHINGTON (dpa-AFX) - Teleflex Incorporated (TFX), Wednesday announced U.S. Food and Drug Administration 510(k) clearance of an expansion to the Indications for Use of the QuikClot Control+ Hemostatic Device to include all grades of internal and external bleeding.
The company added that the expanded indication would help to take effective control of bleeding during treatment, offering support in procedures related to trauma, general surgery, gynecologic surgery, orthopedic surgery, and other areas.
The approval was based on real-world evidence from a retrospective, statistically powered, observational study of 603 emergency, trauma, and surgical patients.
Currently, the QuikClot Control+ Hemostatic Device is indicated for temporary control of external and identifiable sites of internal mild, moderate, severe, and life-threatening bleeding in the U.S.
Meanwhile, the same is indicated for temporary control of internal and external mild, moderate, severe, and traumatic bleeding due to injuries and/or surgical wounds in the European Union, where the device was commercialized in 2024.
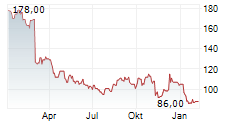
In the pre-market hours, Teleflex's stock is trading at $138.72, up 0.36 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News