BEIJING (dpa-AFX) - Connect Biopharma Holdings Limited (CNTB), Tuesday announced clinical and preclinical data regarding rademikibart for the treatment of patients with asthma or COPD experiencing an acute exacerbation.
The findings from Phase 2b trial revealed that rademikibart improved lung function or FEV within 24 hours, and reduced acute exacerbations in patients with type 2 inflammatory asthma.
Meanwhile, rademikibart significantly improved prebronchodilator FEV from first assessment and was sustained through 24 weeks of treatment, with greatest improvements in patients with elevated baseline eosinophil counts.
Upon comparing Phase 2b trial of rademikibart and from the Phase 3 VENTURE and QUEST trials of dupilumab, the company noted that eosinophil counts decreased by approximately 30 percent at Week 24 in the rademikibart treatment groups, compared to a mean eosinophil increase between 50 percent and 120 percent for dupilumab during 52 weeks of treatment.
Also, molecular dynamics studies showed rademikibart forms a very strong and stable interaction with IL-4R?, confirmed structurally by lower B-factors and more hydrogen bonds than dupilumab.
The findings support Phase 2 acute exacerbation studies in asthma and COPD which are expected to report topline data in first half of 2026.
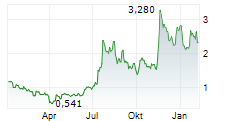
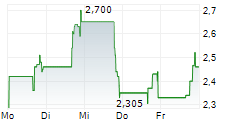
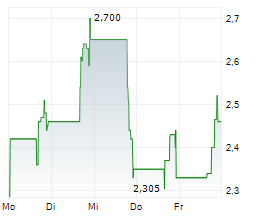
Currently, CNTB is trading at $0.78, down 0.62 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News