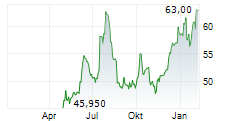
Basilea Pharmaceutica has announced the launch of Zevtera, its lead anti-bacterial treatment, in the US by partner Innoviva Specialty Therapeutics (IST), marking a key strategic milestone for the company. Zevtera, an advanced generation IV cephalosporin antibiotic, was approved by the FDA in April 2024 for three separate indications, including the highly aggressive Staphylococcus aureus bloodstream (SAB) infection. IST was signed as the US licensing partner in December on attractive deal terms, including an upfront payment of US$4m, up to US$223m in sales-related milestone payments as well as tiered royalties (high-teens to mid-20s percent of sales). The US is the most important market for Zevtera, accounting for c 85% of the drug's commercial opportunity, and we expect this launch to boost top-line performance over the coming years. Zevtera holds 10-year market exclusivity in the US and we project peak sales potential of c US$300m in SAB infections in the US.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research