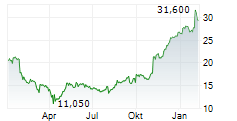
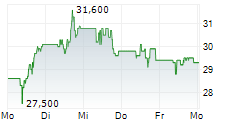
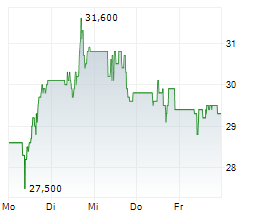
PETAH TIKVA (dpa-AFX) - Teva Pharmaceutical Industries Ltd. (TEVA) announced that the US Food and Drug Administration granted Fast Track designation for investigational TEV-53408, an anti-IL-15 antibody, for the treatment of people with celiac disease on a gluten-free diet.
TEV-53408 is currently being evaluated in a Phase 2a trial to assess the efficacy and safety in adults with celiac disease.
Fast Track is an FDA process designed to facilitate development and expedite review of drugs to treat serious conditions and address unmet medical needs.
TEV-53408 is an antibody designed to inhibit the activity of the cytokine, interleukin-15 (IL-15), to prevent intestinal damage and associated symptoms in individuals with celiac disease.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News