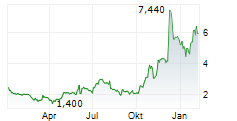
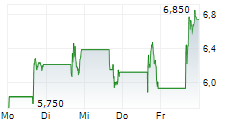
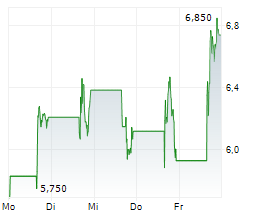
Immix Biopharma has announced a positive clinical update for the US-based Phase I/II NEXICART-2 trial, investigating lead CAR-T candidate NXC-201 as a potential treatment for relapsed/refractory (r/r) amyloid light chain amyloidosis (ALA). These interim results show that, at the data cut off of 28 January 2025, five out of seven (71%) patients had shown a complete response (CR). Furthermore, no relapses or safety concerns have been reported to date, consistent with Immix's goal of making NXC-201 the first 'outpatient' CAR-T therapy. We view this latest update as encouraging for the NEXICART-2 trial, though we acknowledge that the data still correspond to a relatively small population. A more detailed presentation is due to be presented at the 2025 American Society of Clinical Oncology Annual Meeting (ASCO 2025) on 3 June. Immix is also hosting a key opinion leader (KOL) event later the same day to discuss clinical experiences with NXC-201, as well as the evolving treatment landscape for r/r ALA. Separately, Immix also announced that NEXICART-2 now has 14 active US sites (10 more than the company's prior update) and that it anticipates completing the trial ahead of schedule.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research