- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 12.02. | Artificial Intelligence Technology Solutions, Inc.: AITX's RAD Confirms Immix, AWS, and Circadian Risk Participation at ISC West 2026 | 390 | Newsfile | Detroit, Michigan--(Newsfile Corp. - February 12, 2026) - Artificial Intelligence Technology Solutions, Inc. (OTCID: AITX) (the "Company"), a global leader in AI-driven security and productivity... ► Artikel lesen | |
| 28.01. | Immix Biopharma, Inc.: Immix Biopharma Receives U.S. FDA Breakthrough Therapy Designation for NXC-201 | 322 | GlobeNewswire (Europe) | - Breakthrough Therapy Designation granted to NXC-201 based on positive NEXICART-2 Phase 2 interim clinical results, presented at the American Society of Hematology (ASH) 2025 annual meeting - - Final... ► Artikel lesen | |
| 09.01. | Immix Biopharma - NXC-201's path to BLA submission | 418 | Edison Investment Research | Immix Biopharma recently reported a positive clinical update for the registrational NEXICART-2 trial, evaluating lead CAR-T candidate NXC-201 in relapsed/refractory amyloid light chain amyloidosis (r/r... ► Artikel lesen | |
| 10.12.25 | Immix Biopharma stock price target raised to $12 from $8 at H.C. Wainwright | 2 | Investing.com | ||
| 10.12.25 | Immix Biopharma closes $93.7 million public offering | 4 | Investing.com | ||
| 09.12.25 | Immix Biopharma, Inc.: Immix Biopharma Announces Closing of Upsized $100 Million Underwritten Offering of Common Stock and Pre-Funded Warrants | 13 | GlobeNewswire (USA) | ||
| 08.12.25 | Immix Biopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 08.12.25 | Immix Biopharma prices $100M equity raise via common stock and warrant offering | 3 | Seeking Alpha | ||
| IMMIX BIOPHARMA Aktie jetzt für 0€ handeln | |||||
| 08.12.25 | Immix Biopharma prices $100 million public offering to fund AL amyloidosis drug | 2 | Investing.com | ||
| 08.12.25 | Immix Biopharma reports 75% complete response rate for NXC-201 | 1 | Investing.com | ||
| 08.12.25 | Immix Biopharma, Inc.: Immix Biopharma Announces Pricing of Upsized $100 Million Underwritten Offering of Common Stock and Pre-Funded Warrants | 171 | GlobeNewswire (Europe) | LOS ANGELES, CA, Dec. 07, 2025 (GLOBE NEWSWIRE) -- Immix Biopharma, Inc. ("ImmixBio", "Company", "We" or "Us" or "IMMX"), a global leader in relapsed/refractory AL Amyloidosis, today announced the... ► Artikel lesen | |
| 08.12.25 | Immix Biopharma, Inc.: At ASH 2025 Oral Presentation, Immix Biopharma Reports Positive Phase 2 NXC-201 Results, Advancing Toward BLA Submission as a Potentially First- and Best-in-Class Therapy for relapsed/refractory AL Amyloidosis | 137 | GlobeNewswire (Europe) | - NXC-201 demonstrated a complete response (CR) rate of 75% (15/20) (at s/u IFE(-) level) by independent review committee - - In four out of five pending patients, MRD negativity in bone marrow predicts... ► Artikel lesen | |
| 26.11.25 | Immix Biopharma reicht aktualisierte Prüferzustimmungen für SEC-Registrierungserklärung ein | 3 | Investing.com Deutsch | ||
| 26.11.25 | Immix Biopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 12.11.25 | Immix Biopharma appoints Michael Grabow as chief commercial officer to drive NXC-201 launch | 1 | Seeking Alpha | ||
| 12.11.25 | Immix Biopharma appoints commercial leader to head NXC-201 launch | 3 | Investing.com | ||
| 12.11.25 | Immix Biopharma beruft Commercial-Leiter für Markteinführung von NXC-201 | 1 | Investing.com Deutsch | ||
| 12.11.25 | Immix Biopharma, Inc.: Immix Biopharma Appoints Chief Commercial Officer to Drive NXC-201 Launch | 372 | GlobeNewswire (Europe) | - Former Chimerix (CMRX, acquired by Jazz for $935 million) Senior Commercial Leader Michael Grabow led Go-To-Market Strategy and Launch for rare disease therapeutic MODEYSO® - - Rare disease commercial... ► Artikel lesen | |
| 07.11.25 | Immix Biopharma, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 03.11.25 | Immix Biopharma to present NXC-201 trial data for AL amyloidosis at ASH | 2 | Investing.com |
1 2 3 Weiter >>
41 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| QIAGEN | 41,960 | +1,38 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| CRISPR THERAPEUTICS | 50,50 | -0,98 % | Crispr Therapeutics gains amid takeover speculation | ||
| NANOREPRO | 1,535 | -1,92 % | NanoRepro: Neues Geschäftsmodell - mit Kaltplasma | Schnelltests waren lange das Kerngeschäft der NanoRepro AG (ISIN: DE0006577109). Spätestens seit dem Ende des Corona-Booms war klar, dass das Unternehmen seinen Kurs neu ausrichten muss. Was seitdem... ► Artikel lesen |
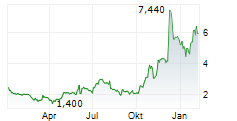
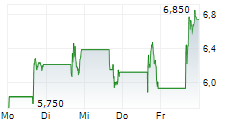
IMMIX BIOPHARMA INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,425 (-5,01 %) liegt der Kurs bei 8,065 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu IMMIX BIOPHARMA INC finden Sie auf Wallstreet Online.