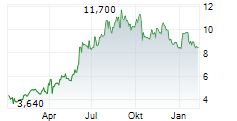
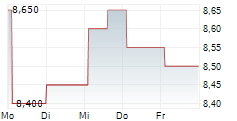
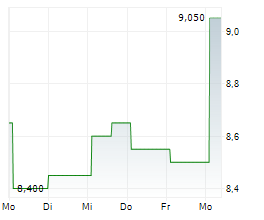
BEIJING (dpa-AFX) - Innovent Biologics Inc. (IVBXF.OB) announced Wednesday that the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) has granted a second Breakthrough Therapy Designation to its first-in-class PD-1/IL-2?-bias bispecific antibody fusion protein, IBI363, for the treatment of unresectable, locally advanced, or metastatic squamous non-small cell lung cancer or sqNSCLC that has progressed following anti-PD-(L) immunotherapy and platinum-based chemotherapy.
To date, IBI363 has received Breakthrough Therapy Designations from China's NMPA CDE and Fast Track Designations from the U.S. FDA for two indications-sqNSCLC and melanoma. The latest Breakthrough Therapy Designation further advances IBI363's potential in addressing immunotherapy resistance and cold tumor challenges.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News