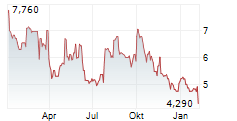
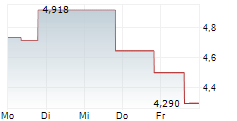
OSE has announced plans to strengthen its growth strategy and accelerate the key pillars of its pipeline, covering immuno-inflammation and immuno-oncology. In the inflammation space, the company revealed a potential path forward for lusvertikimab, which reported positive top-line data in Phase II (CoTikiS) for ulcerative colitis (UC) in Q424. A new predictive biomarker (a composite IL7R axis biomarker) has been identified through a retrospective analysis of CoTikiS, offering potential to improve clinical remission rates through a precision medicine approach with lusvertikimab, for the biomarker-positive population. Management is preparing for a Phase IIb programme to validate this biomarker, with the aim of demonstrating efficacy in this population by 2027. If successful, this should lay the foundation for late-stage clinical development efforts for lusvertikimab. In the oncology space, the latest update confirmed that the company is on track with its plans for regulatory submission, with the pivotal Phase III non-small cell lung cancer trial due to conclude in 2027, with potential to expand the label to other indications (such as pancreatic cancer) in later years, contingent on successful clinical progression.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research