| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
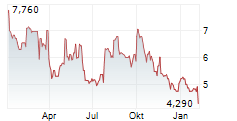
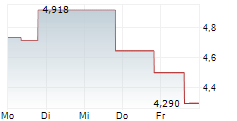
| 4,228 | 4,338 | 01.03. | |
| 4,300 | 4,340 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | OSE Immunotherapeutics Receives Second Positive IDMC Recommendation for Phase 3 ARTEMIA Trial Evaluating Tedopi in Non-Small Cell Lung Cancer | 364 | GlobeNewswire (Europe) | NANTES, France, February 26, 2026 - 6pm CET - OSE Immunotherapeutics SA (ISIN: FR0012127173; Mnemo: OSE), today announced that the Independent Data Monitoring Committee (IDMC) has issued a second... ► Artikel lesen | |
| 29.01. | OSE Immunotherapeutics Selects Chronic Pouchitis and Hidradenitis Suppurativa as New Key Indications for Lusvertikimab | 125 | GlobeNewswire (Europe) | OSE Immunotherapeutics Selects Chronic Pouchitis and Hidradenitis Suppurativa as New Key Indications for Lusvertikimab Development built on strong IL-7R biological rationaleChronic Pouchitis offers... ► Artikel lesen | |
| 21.01. | OSE Immunotherapeutics Welcomes FDA Orphan Drug Designation Granted to Pegrizeprument (VEL-101) | 383 | GlobeNewswire (Europe) | OSE Immunotherapeutics Welcomes FDA Orphan Drug Designation Granted to Pegrizeprument (VEL-101) Nantes, France, January 21, 2026 - 6pm CET - OSE Immunotherapeutics SA (ISIN: FR0012127173; Mnemo: OSE)... ► Artikel lesen | |
| 20.01. | OSE Immunotherapeutics: Half-Year Report on Liquidity Contract with Invest Securities | 11 | GlobeNewswire (USA) | ||
| 09.01. | OSE Immunotherapeutics - Renewed focus on advancing core assets | 386 | Edison Investment Research | We revisit our investment case for OSE Immunotherapeutics following the recently presented strategic plans for 2026-28, reflecting a more streamlined focus on advancing its core clinical assets, Tedopi... ► Artikel lesen | |
| OSE IMMUNOTHERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 09.12.25 | OSE Immunotherapeutics - Strategic plan presented for 2026-2028 | 380 | Edison Investment Research | OSE has announced its strategic plan for the next three years, which is designed to create multiple near-term catalysts. Importantly, it aims to extract maximum value from its lead proprietary candidates... ► Artikel lesen | |
| 09.12.25 | OSE Immunotherapeutics Unveils 2026-2028 Strategic Plan With Four Opportunities for Value Creation | 6 | GlobeNewswire (USA) | ||
| 08.12.25 | AbbVie-Deal belastet: Aktie von Ose Immunotherapeutics gibt nach | 22 | Investing.com Deutsch | ||
| 08.12.25 | OSE Immunotherapeutics Announces Strategic Amendment to AbbVie's Partnership on ABBV-230 Development | 175 | GlobeNewswire (Europe) | OSE Immunotherapeutics Announces Strategic Amendment to AbbVie's Partnership on ABBV-230 Development Strategic Amendment Enhances OSE Immunotherapeutics' Role in ABBV-230 Development While Preserving... ► Artikel lesen | |
| 17.11.25 | OSE Immunotherapeutics Announces Positive Recommendation from Independent Data Monitoring Committee (IDMC) to Continue Pivotal Phase 3 ARTEMIA Trial Evaluating Tedopi in Non-Small Cell Lung Cancer | 346 | GlobeNewswire (Europe) | OSE Immunotherapeutics Announces Positive Recommendation from Independent Data Monitoring Committee (IDMC) to Continue Pivotal Phase 3 ARTEMIA Trial Evaluating Tedopi® in Non-Small Cell Lung Cancer... ► Artikel lesen | |
| 07.11.25 | OSE Immunotherapeutics Presents a Poster on OSE-Cytomask, a Novel "Cis-Demasking" Cytokine Technology to Increase Therapeutic Index of Immunocytokines | 1 | GlobeNewswire (USA) | ||
| 20.10.25 | OSE Immunotherapeutics - Refocusing for the next phase | 359 | Edison Investment Research | OSE Immunotherapeutics' H125 results, the first since the board restructuring, reflected normalised operations typical of a clinical-stage biotech, following H124's exceptional licensing income. The... ► Artikel lesen | |
| 15.10.25 | OSE Immunotherapeutics Reports First Half 2025 Financial Results | 472 | GlobeNewswire (Europe) | OSE Immunotherapeutics Reports First Half 2025 Financial Results NANTES, France, October 15, 2025 - 7:00pm CET - OSE Immunotherapeutics SA (ISIN: FR0012127173; Mnemo: OSE), today reported its consolidated... ► Artikel lesen | |
| 03.10.25 | OSE ImmunoTherapeutics replaces CEO Nicolas Poirier, appoints Marc Le Bozec as interim CEO | 3 | Seeking Alpha | ||
| 03.10.25 | OSE Immunotherapeutics Names Marc Le Bozec Interim CEO, Effective Immediately | 1 | RTTNews | ||
| 03.10.25 | OSE Immunotherapeutics appoints Marc Le Bozec as interim CEO | 10 | Investing.com | ||
| 03.10.25 | OSE Immunotherapeutics beruft Marc Le Bozec zum Interims-CEO | 2 | Investing.com Deutsch | ||
| 03.10.25 | OSE Immunotherapeutics: Appointment of Marc Le Bozec as Interim CEO | 184 | GlobeNewswire (Europe) | Appointment of Marc Le Bozec as Interim CEO Nantes, October 3, 2025, 8:00 a.m. CET - OSE Immunotherapeutics (ISIN: FR0012127173; Mnemonic: OSE) announces the termination of the position as chief... ► Artikel lesen | |
| 30.09.25 | OSE Immunotherapeutics: Full renewal of the Board of Directors Election of Dr. Markus Cappel as Chairman of the Board of Directors | 180 | GlobeNewswire (Europe) | Full renewal of the Board of DirectorsElection of Dr. Markus Cappel as Chairman of the Board of Directors Nantes, September 30, 2025, 7:30 p.m. CET - OSE Immunotherapeutics (ISIN: FR0012127173; Mnemonic:... ► Artikel lesen | |
| 26.09.25 | OSE Immunotherapeutics - Updated cash position; new collaboration | 449 | Edison Investment Research | While OSE Immunotherapeutics' H125 results were previously due to be presented in September, they are now scheduled for 15 October 2025 to allow for the AGM (30 September 2025) to take place ahead of... ► Artikel lesen |
1 2 3 Weiter >>
52 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning |
Das Unternehmen OSE IMMUNOTHERAPEUTICS SA kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell -0,28 % bei einem Aktienkurs von 4,310 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu OSE IMMUNOTHERAPEUTICS SA finden Sie auf Wallstreet Online.