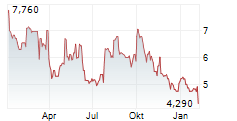
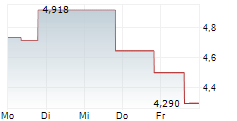
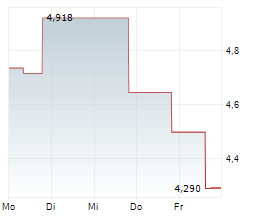
OSE has announced its strategic plan for the next three years, which is designed to create multiple near-term catalysts. Importantly, it aims to extract maximum value from its lead proprietary candidates, Tedopi and lusvertikimab. For Tedopi, the registrational Phase III ARTEMIA trial in non-small cell lung cancer remains on track to report a futility analysis in Q326, followed by a full readout in Q128. For lusvertikimab, while OSE was previously planning a Phase IIb trial and precision medicine approach in ulcerative colitis (UC), the company now plans to target less capital-intensive indications, such as speciality/rare diseases; further details will be disclosed in early 2026. Management has also communicated it will seek partnership opportunities for the candidate, with the aim of finding a partner that is equipped to efficiently advance the candidate in UC, following encouraging prior results in Phase II. OSE has guided a cash runway to Q426 (conservatively excluding potential financing or milestone payments from partners). While existing and potential new partnerships may reduce financing requirements, we acknowledge the possibility OSE may need to utilise alternative avenues of capital.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research