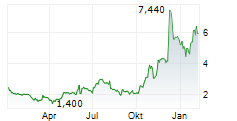
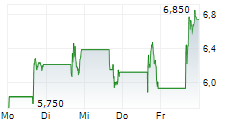
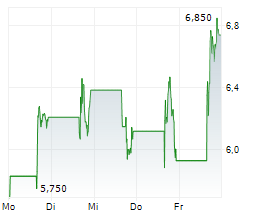
Following Immix's update at the 61st annual American Society of Clinical Oncology conference (ASCO 2025), the company hosted a key opinion leader (KOL) event, which showcased the potential of NXC-201 as an effective treatment option to address the unmet need for patients with relapsed/refractory amyloid light chain amyloidosis (r/r ALA). NXC-201 is the only CAR-T therapy in development for ALA, to our knowledge, offering a glimmer of hope to a patient population that currently only has limited treatment options and for which there are no FDA-approved drugs. The KOLs (three US-based physicians) expressed a willingness to treat patients who do not respond to first-line ALA treatment and those ineligible for stem cell transplants with NXC-201, based on the encouraging clinical data to date. The latest clinical update (NEXICART-2 data presented at ASCO 2025) showed a complete response (CR) rate of 70%, with an attractive safety profile, consistent with prior clinical readouts.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research