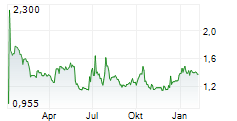
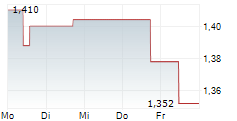
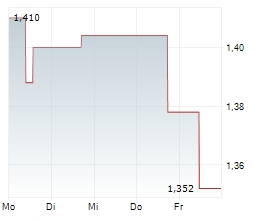
PARIS (dpa-AFX) - AB Science (ABSCF.PK) announced that the European Medicines Agency (EMA) has approved an extension of the shelf life of its veterinary medicine, MASIVET, to 48 months from 36 months. This applies specifically to the 50mg tablet version available in the European Union.
MASIVET is used to treat non-resectable mast cell tumors in dogs (Grade 2 or 3) with confirmed mutations in the c-kit tyrosine kinase receptor. The shelf-life extension allows greater flexibility in inventory management, helping patients, caregivers, and veterinarians maintain treatment availability while reducing the risk of product expiration. This change supports continued access to effective therapies across all EU countries.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News