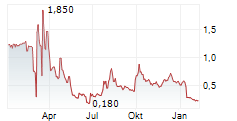
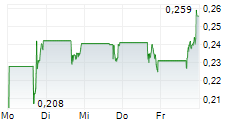
WASHINGTON (dpa-AFX) - Plus Therapeutics, Inc. (PSTV), a clinical-stage pharmaceutical company, expects the revenue contributions of CNSide Diagnostics, LLC to 'become meaningful' in fiscal year 2026.
The proprietary CNSide Cerebrospinal Fluid (CSF) Assay Platform is designed and intended for patients suspected of having central nervous system cancer metastases (CNS Mets). The first test to be commercialized, CNSide CSF Tumor Cell Enumeration (TCE), has a total addressable market estimated to be $6 billion in the U.S.
Andrew Sims, Plus Therapeutics' Vice President and Chief Financial Officer. 'We anticipate that while CNSide's launch is on track for 2025, given our current forecasts the revenue contributions of the CNSide subsidiary will become meaningful to Plus Therapeutics' operations in fiscal year 2026.'
CNS Mets are an epidemic affecting as many as 30% of adult cancer patients and affect the highly protected CNS space. Once the CNS is affected, diagnosis and treatment are difficult and as a result, approximately half of patients with CSF metastases instead receive just palliative care or hospice. The current standard of care for CNS Mets diagnosis, CSF cytology, was developed over a century ago and offers suboptimal test sensitivity leading to missed or delayed diagnosis and treatment.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News