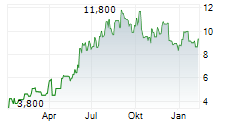
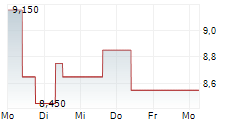
BEIJING (dpa-AFX) - Innovent Biologics, Inc. (IVBXF.OB) announced Friday that China's National Medical Products Administration or NMPA has approved mazdutide for chronic weight management in Chinese adults with overweight or obesity.
Mmazdutide is a first-in-class dual glucagon (GCG)/glucagon-like peptide-1 (GLP-1) receptor agonist.
The biopharmaceutical company focused on oncologic, autoimmune, cardiovascular and metabolic, ophthalmologic and other major diseases said the approval of mazdutide was mainly based on data from GLORY-1, a Phase 3 pivotal clinical study conducted in Chinese adults with overweight or obesity.
In the trial, the primary endpoint and all key secondary endpoints of the study were successfully achieved in 2024.
The company noted that Mazdutide is the world's first dual GCG/GLP-1 receptor agonist approved for weight loss. It was named by Fierce Pharma as one of the top ten most anticipated drugs globally in 2025.
Mazdutide is supported by robust clinical data published in multiple high-impact journals, including Nature, the Lancet sub-journals, and the New England Journal of Medicine.
Being the first marketed dual GCG/GLP-1 receptor agonist, mazdutide has been recommended by multiple clinical guidelines in China and expert consensus on obesity management on account of its innovative mechanism and solid evidence base, the company said.
Professor Linong Ji, the Leading Principal Investigator of GLORY-1, Peking University People's Hospital, stated, 'Obesity is a chronic disease that demands a coordinated societal response. With China facing a high prevalence of overweight and obesity, the associated cardiometabolic disease burdens continue to rise. There is an urgent need for weight-loss therapies that are both effective and safe, with proven cardiovascular and metabolic benefits. As the principal investigator of this novel dual GCG/GLP-1 receptor agonist with a unique mechanism of action, I'm proud to see our clinical results recognized by China's national regulatory authority and anticipate its subsequent approval for market launch. My fellow researchers and I hope mazdutide will become a valuable therapeutic option for Chinese adults with overweight or obesity.'
Mazdutide currently has another NDA accepted for review by NMPA, for glycemia control in adults with type 2 diabetes.
For More Such Health News, visit rttnews.com
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News