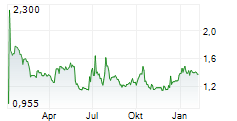
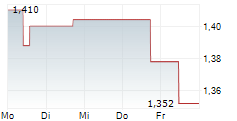
PARIS (dpa-AFX) - AB Science (ABSCF.PK) announced FDA and EMA authorization of a confirmatory Phase 3 trial of masitinib in metastatic castrate-resistant prostate cancer, using a harmonized protocol and biomarker targeting patients with less advanced disease.
The Study is a prospective, multicenter, randomized, double blind, placebo-controlled, 2-parallel groups, phase 3 study to confirm the efficacy and safety of docetaxel (IV 75 mg/m plus prednisone for up to 10 cycles) plus masitinib 6.0 mg/kg/d, versus docetaxel plus placebo in metastatic castrate resistant prostate cancer (mCRPC).
The company noted that the study will enroll 600 patients with confirmed mCRPC eligible to docetaxel and with a biomarker (as measured by baseline alkaline phosphatase level) indicative of less advanced metastatic disease. The study's primary endpoint will be radiographic progression free survival (rPFS), supported by overall survival as the first secondary endpoint.
AB Science filed a patent application relating to methods of treating mCRPC (i.e. a secondary medical use patent) with its lead compound masitinib.
The European Patent Office has granted this patent. It provides protection until 2042 for masitinib and related compounds for treatment of mCRPC in the patient subpopulation with low metastatic involvement, which is the patient population in the approved phase 3 study of masitinib in mCRPC.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News