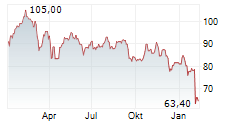
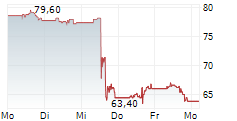
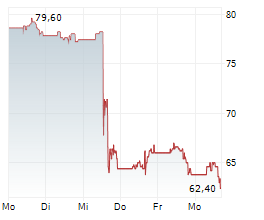
WASHINGTON (dpa-AFX) - Boston Scientific (BSX) has received FDA approval to expand the instructions for use labeling for the FARAPULSE Pulsed Field Ablation System. The updated labeling now includes approval for the system in the treatment of drug refractory, symptomatic persistent atrial fibrillation, an arrythmia in which the heart beats abnormally for at least seven days.
The company said FDA approval for expanded labeling was supported by clinical evidence from phase one of the ADVANTAGE AF clinical trial. Boston Scientific expects CE mark as well as approval in Japan and China in the coming months.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News