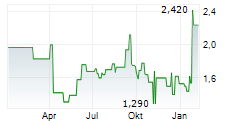
BEIJING (dpa-AFX) - Adagene Inc. (ADAG) Tuesday said it has received feedback from the United States Food and Drug Administration (FDA) on its clinical development plan to evaluate muzastotug in combination with Merck's cancer immunotherapy drug Keytruda in patients with microsatellite stable colorectal cancer (MSS CRC).
The company plans to begin enrolling patients in Phase 2 study of muzastotug in the second half of this year.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News