WASHINGTON (dpa-AFX) - Becton, Dickinson and Company (BDX), Wednesday announced that its BD Veritor System for SARS-CoV-2, a digital test designed to detect COVID-19 antigens in symptomatic individuals in about 15 minutes, has received U.S. Food and Drug Administration 510(k) clearance.
The test can be taken at doctors' offices, urgent care centers, retail clinics and other convenient points of care, with results provided within six days of symptom onset, helping clinicians make confident and timely decisions.
Previously, the test has been available under Emergency Use Authorization or EUA from the FDA. However, the latest clearance will eventually replace the current EUA version of the test, starting in early fall 2025.
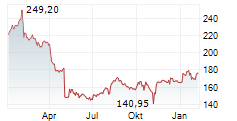
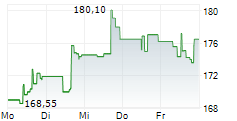
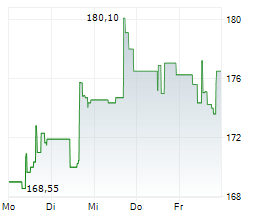
In the pre-market hours, BDX is trading at $183.2, up 0.45 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News