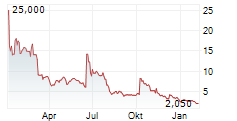
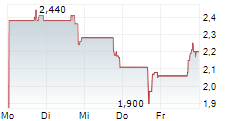
Acurx's common stock is expected to begin trading on a post-split adjusted basis on August 5, 2025
STATEN ISLAND, N.Y., July 31, 2025 /PRNewswire/ -- Acurx Pharmaceuticals, Inc. (NASDAQ: ACXP) ("Acurx" or the "Company"), a late-stage biopharmaceutical company developing a new class of antibiotics for difficult-to-treat bacterial infections, today announced that the board of directors of the Company approved a 1-for-20 reverse stock split (the "Reverse Split") of the Company's common stock. The Reverse Split was approved by the stockholders at the Company's annual meeting of the stockholders held on July 17, 2025. The Reverse Split will legally take effect at 4:01 p.m. Eastern Time, on August 4, 2025. The Company's common stock will open for trading under a new CUSIP number 00510M 203 on The Nasdaq Capital Market on August 5, 2025, on a split-adjusted basis under the current ticker symbol "ACXP." The Reverse Split is intended to increase the per share trading price of the Company's common stock to enable the Company to regain compliance with the minimum bid price requirement for continued listing on The Nasdaq Capital Market.
The 1-for-20 Reverse Split will automatically convert every twenty (20) current shares of the Company's common stock into one (1) share of common stock. No fractional shares will be issued in connection with the Reverse Split. Stockholders who would otherwise hold a fractional share of the Company's common stock following the Reverse Split will receive a cash payment in lieu thereof equal to the fractional share to which the stockholder would otherwise be entitled multiplied by the closing sales price of a share of the Company's common stock on The Nasdaq Capital Market, as adjusted for the Reverse Split, on the trading day immediately prior to the effective date of the Reverse Split, August 1, 2025.
The Reverse Split will reduce the number of shares of outstanding common stock from approximately 30,764,540 shares, the number of shares outstanding as of July 24, 2025, to approximately 1,538,227 shares. The total authorized number of shares will not be reduced. Proportional adjustments will also be made to the exercise and conversion prices of the Company's outstanding stock options and warrants, and to the number of shares issued and issuable under the Company's stock incentive plans.
Stockholders holding their shares electronically in book-entry form are not required to take any action to receive post-split shares. Stockholders owning shares through a bank, broker, or other nominee will have their positions automatically adjusted to reflect the Reverse Split, subject to brokers' particular processes, and will not be required to take any action in connection with the Reverse Split. For those stockholders holding physical stock certificates, the Company's transfer agent, VStock Transfer, LLC, will send instructions for exchanging those certificates for shares held electronically in book-entry form or for new certificates, in either case representing the post-split number of shares, and any payments in cash in lieu of fractional shares, if applicable.
About Acurx Pharmaceuticals, Inc.
Acurx Pharmaceuticals is a late-stage biopharmaceutical company focused on developing a new class of small molecule antibiotics for difficult-to-treat bacterial infections. The Company's approach is to develop antibiotic candidates with a Gram-positive selective spectrum (GPSS®) that blocks the active site of the Gram+ specific bacterial enzyme DNA polymerase IIIC (pol IIIC), inhibiting DNA replication and leading to Gram-positive bacterial cell death. Its R&D pipeline includes antibiotic product candidates that target Gram-positive bacteria, including Clostridioides difficile, methicillin- resistant Staphylococcus aureus (MRSA), vancomycin resistant Enterococcus (VRE), drug- resistant Streptococcus pneumoniae (DRSP) and B. anthracis (anthrax; a Bioterrorism Category A Threat-Level pathogen). Acurx's lead product candidate, ibezapolstat, for the treatment of C. difficile Infection is Phase 3 ready with plans in progress to begin international clinical trials next year subject to obtaining appropriate financing. The Company's preclinical pipeline includes development of an oral product candidate for treatment of ABSSSI (Acute Bacterial Skin and Skin Structure Infections), upon which a development program for treatment of inhaled anthrax is being planned in parallel.
To learn more about Acurx Pharmaceuticals and its product pipeline, please visit www.acurxpharma.com.
Forward Looking Statements
Any statements in this press release about our future expectations, plans and prospects, including statements regarding our strategy, future operations, prospects, plans and objectives, the timing and effectiveness of the Reverse Split, the Company's ability to regain compliance with the Nasdaq minimum bid price and other listing requirement and other statements containing the words "believes," "anticipates," "plans," "expects," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: whether ibezapolstat will benefit from the QIDP designation; whether ibezapolstat will advance through the clinical trial process on a timely basis; whether the results of the clinical trials of ibezapolstat will warrant the submission of applications for marketing approval, and if so, whether ibezapolstat will receive approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies where approval is sought; whether, if ibezapolstat obtains approval, it will be successfully distributed and marketed; and other risks and uncertainties described in the Company's annual report filed with the Securities and Exchange Commission on Form 10-K for the year ended December 31, 2024, and in the Company's subsequent filings with the Securities and Exchange Commission. Such forward- looking statements speak only as of the date of this press release, and Acurx disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances after the date of such statements, except as may be required by law.
Investor Contact:
Acurx Pharmaceuticals, Inc.
David P. Luci, President & Chief Executive Officer
Tel: 917-533-1469
Email: [email protected]
SOURCE Acurx Pharmaceuticals, Inc.