GRAND CAYMAN, Cayman Islands, Aug. 12, 2025 (GLOBE NEWSWIRE) -- Silexion Therapeutics Corp. (NASDAQ: SLXN) ("Silexion" or the "Company"), a clinical-stage biotechnology company developing RNA interference (RNAi) therapies for KRAS-driven cancers, today reported its financial results for the second quarter ended June 30, 2025, and provided an update on recent business developments.
Recent Milestones & Business Highlights:
Groundbreaking Preclinical Data Across Multiple Cancer Types: Following the completion of studies evaluating the Company's second-generation drug candidate SIL204 in orthotopic pancreatic cancer models in February 2025, the Company announced a significant expansion of its preclinical program. In May 2025, Silexion announced preclinical studies exploring SIL204's potential impact on colorectal and lung cancer, with results demonstrating strong efficacy across multiple KRAS-driven cancer types:
- Pancreatic Cancer: SIL204 demonstrated up to 94% inhibition in cancer cells harboring KRAS G12D mutations
- Colorectal Cancer: Achieved approximately 90% inhibition rate in GP2D colorectal cancer cells with KRAS G12D mutations
- Lung Cancer: Showed significant dose-dependent inhibition in human lung cancer cell lines harboring KRAS G12D mutations
- New KRAS Mutation Coverage: In July 2025, the Company reported first evidence of SIL204's efficacy against the clinically significant KRAS Q61H mutation, with up to 97% inhibition in pancreatic cancer cells
Strategic Formulation Partnership: In April 2025, Silexion announced a strategic collaboration with Catalent, a global leader in advanced delivery technologies, for formulation development and clinical manufacturing activities for SIL204 at Catalent's facility in Limoges, France. This partnership supports the Company's dual-route development strategy targeting both the primary tumor and resulting metastases.
Regulatory and Clinical Timeline: The Company remains on track to initiate Phase 2/3 clinical trials for SIL204 in the first half of 2026, focusing initially on locally advanced pancreatic cancer.
Silexion is currently conducting the toxicology studies to initiate the clinical trial, and those are progressing as planned. Silexion plans for regulatory submission to initiate the Phase 2/3 trial to the Israel Ministry of Health in the fourth quarter of 2025 and the European Union in the first quarter of 2026.
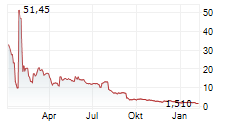
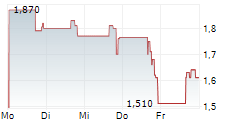
Nasdaq Listing Maintained: In July 2025, the Company received a favorable decision from a Nasdaq hearings panel, allowing it to maintain its listing on the Nasdaq Capital Market. As part of maintaining compliance, Silexion effected a 1-for-15 reverse share split on July 29, 2025, and continues to work toward achieving the required $2.5 million shareholders' equity threshold.
Recent Financing Activities: Subsequent to the quarter end, on July 31, 2025, the Company entered into a warrant exercise inducement transaction generating approximately $1.8 million in gross proceeds from the exercise of 152,106 existing warrants.
Ilan Hadar, Chairman and CEO of Silexion commented: "The second quarter of 2025 marked a transformative period for Silexion as we significantly expanded the potential therapeutic applications of SIL204 beyond pancreatic cancer. Our preclinical data now demonstrates SIL204's impressive efficacy across pancreatic, colorectal, and lung cancers - three of the most challenging KRAS-driven malignancies. With high inhibition rates and coverage of multiple KRAS mutations including the newly validated Q61H and G13D variants, SIL204 is positioned as a potentially transformative pan-KRAS therapeutic."
"The strategic collaboration with Catalent and our continued progress toward clinical trials in 2026 reinforce our commitment to bringing this innovative RNAi therapy to patients with limited treatment options. As we advance our dual-route administration strategy and maintain our Nasdaq listing, we remain focused on executing our clinical development plan and delivering value to both patients and shareholders."
Second Quarter 2025 Financial Results:
Cash Position: Cash and cash equivalents were $3.5 million as of June 30, 2025, compared to $1.2 million as of December 31, 2024. The increase primarily reflects proceeds from financing activities completed in early 2025, partially offset by ongoing operational expenses supporting preclinical development activities.
Operating Expenses: Total operating expenses for the three-month period ended June 30, 2025 were $2.3 million, compared to $1.4 million for the three-month period ended June 30, 2024, an increase of 64.3%. Research and development expenses increased to $1.0 million for the three-month period ended June 30, 2025, compared to $0.8 million for the three-month period ended June 30, 2024, an increase of $0.2 million or 25.0%, primarily due to increased payroll and payroll-related expenses from additional headcount and salary increases following the business combination, as well as bonus accrual in the second quarter of 2025. General and administrative expenses increased to $1.3 million for the three-month period ended June 30, 2025, compared to $0.6 million for the three-month period ended June 30, 2024, an increase of $0.7 million or 116.7%, mainly due to increased payroll expenses and professional services costs associated with operating as a public company.
Financial Expenses: Financial expenses, net for the three-month period ended June 30, 2025 were $0.2 million, compared to $0.1 million for the three-month period ended June 30, 2024, an increase of $0.1 million or 100.0%, primarily due to an increase in revaluation expenses of financial instruments.
Net Loss: Net loss for the three-month period ended June 30, 2025 was $2.5 million, compared to $1.5 million for the three-month period ended June 30, 2024, an increase of $1.0 million or 66.7%. The increase was mainly due to an increase in our general and administrative expenses related to our status as a public company and research and development expenses.
Six-Month Results: For the six months ended June 30, 2025, net loss was $4.2 million compared to $2.9 million for the same period in 2024, primarily reflecting increased general and administrative expenses related to operating as a public company.
Notice Regarding Forward-Looking Statements:
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion's business strategy, preclinical and clinical development plans, timeline for clinical trials, regulatory submissions, collaboration with Catalent, and expectations regarding SIL204's therapeutic potential, are forward-looking statements. These forward-looking statements are generally identified by terminology such as "may", "should", "could", "might", "plan", "possible", "project", "strive", "budget", "forecast", "expect", "intend", "will", "estimate", "anticipate", "believe", "predict", "potential" or "continue", or the negatives of these terms or variations of them or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied in those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion's ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion's strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion's future capital requirements and sources and uses of cash, including its ability to obtain additional capital; (vi) Silexion's ability to maintain its Nasdaq listing; and (vii) other risks and uncertainties set forth in the documents filed or to be filed with the SEC by the Company, including the Company's Quarterly Report on Form 10-Q for the period ended June 30, 2025. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact:
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
mirit@silexion.com
Capital Markets & IR Contact:
Arx Capital Markets
North American Equities Desk
silexion@arxadvisory.com
| SILEXION THERAPEUTICS CORP UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS | ||
| June 30, 2025 | December 31, 2024 | |
| U.S. dollars in thousands | ||
| Assets | ||
| CURRENT ASSETS: | ||
| Cash and cash equivalents | $3,466 | $1,187 |
| Restricted cash | 25 | 35 |
| Prepaid expenses | 1,683 | 966 |
| Other current assets | 63 | 62 |
| TOTAL CURRENT ASSETS | 5,237 | 2,250 |
| NON-CURRENT ASSETS: | ||
| Restricted cash | 53 | 48 |
| Long-term deposit | 5 | 5 |
| Property and equipment, net | 30 | 30 |
| Operating lease right-of-use asset | 472 | 530 |
| TOTAL NON-CURRENT ASSETS | 560 | 613 |
| TOTAL ASSETS | $5,797 | $2,863 |
| SILEXION THERAPEUTICS CORP UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS | ||
| June 30, 2025 | December 31, 2024 | |
| U.S. dollars in thousands | ||
| Liabilities and shareholders' equity (capital deficiency) | ||
| CURRENT LIABILITIES: | ||
| Trade payables | $692 | $929 |
| Current maturities of operating lease liability | 171 | 158 |
| Employee related obligations | 628 | 642 |
| Accrued expenses and other account payable | 659 | 788 |
| Private warrants to purchase ordinary shares (including $* and $1 due to related party, as of June 30, 2025 and December 31, 2024, respectively) | * | 2 |
| Underwriters Promissory Note | - | 1,004 |
| TOTAL CURRENT LIABILITIES | 2,150 | 3,523 |
| NON-CURRENT LIABILITIES: | ||
| Long-term operating lease liability | 337 | 368 |
| Related Party Promissory Note | 3,190 | 2,961 |
| TOTAL NON-CURRENT LIABILITIES | $3,527 | $3,329 |
| TOTAL LIABILITIES | $5,677 | $6,852 |
| SHAREHOLDERS' EQUITY (CAPITAL DEFICIENCY): | ||
| Ordinary shares ($0.0135 par value per share, 1,481,482 shares authorized as of June 30, 2025 and December 31, 2024; 579,536 and 123,290** shares issued and outstanding as of June 30, 2025 and December 31, 2024, respectively) | 8 | 2 |
| Additional paid-in capital | 47,604 | 39,263 |
| Accumulated deficit | (47,492) | (43,254) |
| TOTAL SHAREHOLDERS' EQUITY (CAPITAL DEFICIENCY) | $120 | $(3,989) |
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY (CAPITAL DEFICIENCY) | $5,797 | $2,863 |
All share amounts have been retroactively adjusted to reflect a 1-for-15 reverse share split as discussed in Note 11(b) to the Company's condensed consolidated financial statements included in the Company's quarterly report on Form 10-Q for the quarter ended June 30, 2025
* Represents an amount less than $1
** Net of 28 treasury shares held by the Company as of December 31, 2024
| SILEXION THERAPEUTICS CORP UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS | ||||
| Six months ended June 30 | Three months ended June 30 | |||
| 2025 | 2024 | 2025 | 2024 | |
| U.S. dollars in thousands | U.S. dollars in thousands | |||
| OPERATING EXPENSES: | ||||
| Research and development (including $0 and $34 from related party for the six months period ended June 30, 2025 and 2024, respectively, and including $0 and $17 from related party for the three months period ended June 30, 2025 and 2024, respectively) | $1,608 | $1,727 | $1,018 | $766 |
| General and administrative (including $58 and $24 from related party for the six months period ended June 30, 2025 and 2024, respectively, and including $37 and $12 from related party for the three months period ended June 30, 2025 and 2024, respectively) | 2,326 | 908 | 1,266 | 619 |
| TOTAL OPERATING EXPENSES | 3,934 | 2,635 | 2,284 | 1,385 |
| OPERATING LOSS | 3,934 | 2,635 | 2,284 | 1,385 |
| Financial expenses, net (including $229 and $135 from related party for the six months period ended June 30, 2025 and 2024, respectively, and including $197 and $60 from related party for the three months period ended June 30, 2025 and 2024, respectively) | 301 | 270 | 216 | 102 |
| LOSS BEFORE INCOME TAX | $4,235 | $2,905 | $2,500 | $1,487 |
| INCOME TAX | 3 | 7 | 3 | 2 |
| NET LOSS | $4,238 | $2,912 | $2,503 | $1,489 |
| Attributable to: | ||||
| Equity holders of the Company | 4,238 | 2,845 | 2,503 | 1,472 |
| Non-controlling interests | - | 67 | - | 17 |
| Total | $4,238 | $2,912 | $2,503 | $1,489 |
| LOSS PER SHARE, BASIC AND DILUTED | $8.21 | $381.09 | $4.32 | $197.80 |
| WEIGHTED AVERAGE NUMBER OF ORDINARY SHARES OUTSTANDING USED IN COMPUTATION OF BASIC AND DILUTED LOSS PER SHARE | 516,110 | 7,466 | 579,523 | 7,442 |
All share and per share amounts have been adjusted (for any period preceding the relevant reverse share split, on a retroactive basis) to reflect 1-for-9 and 1-for-15 reverse share splits, as discussed in Notes 1(g) and 11(b) to the Company's condensed consolidated financial statements included in the Company's quarterly report on Form 10-Q for the quarter ended June 30, 2025