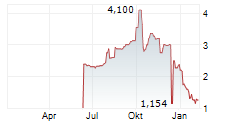
Relief Therapeutics Holding SA / Key word(s): Half Year Results Relief Therapeutics Publishes 2025 Half-Year Report GENEVA (AUG. 14, 2025) - RELIEF THERAPEUTICS Holding SA (SIX: RLF, OTCQB: RLFTF, RLFTY) (Relief, or the Company), a biopharmaceutical company committed to delivering innovative treatment options for select specialty, unmet and rare diseases, today announced the publication of its 2025 half-year report and provided a corporate update. "Relief continued to make steady progress across our pipeline and corporate initiatives," said Dr. Raghuram Selvaraju, chairman of the board of directors. "We advanced RLF-TD011, our lead wound care candidate, with the receipt of Rare Pediatric Disease designation for epidermolysis bullosa and a productive Type B pre-IND meeting with the FDA. We also progressed RLF-OD032, our next-generation liquid sapropterin formulation for phenylketonuria, toward a pivotal trial expected to begin this quarter. Our focus remains on advancing our core programs, with existing cash reserves projected to fund operations into at least late 2026, beyond the targeted regulatory submission of RLF-OD032 for approval in the United States. In parallel, we are taking steps to establish a scalable, AI-driven health tech company through the proposed business combination with NeuroX." The report, available on the Company's website, includes interim financial statements for the six months ended June 30, 2025, and a shareholder update. ABOUT RELIEF CONTACT DISCLAIMER Additional features: File: Ad hoc End of Inside Information |
| Language: | English |
| Company: | Relief Therapeutics Holding SA |
| Avenue de Secheron 15 | |
| 1202 Geneva | |
| Switzerland | |
| Phone: | +41 22 545 11 16 |
| E-mail: | contact@relieftherapeutics.com |
| Internet: | https://relieftherapeutics.com |
| ISIN: | CH1251125998 |
| Valor: | 125112599 |
| Listed: | SIX Swiss Exchange |
| EQS News ID: | 2183666 |
| End of Announcement | EQS News Service |
2183666 14-Aug-2025 CET/CEST