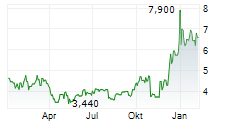
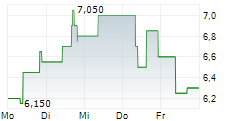
WASHINGTON, Aug. 18, 2025 /PRNewswire/ -- Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today announced that it secured a landmark victory over the U.S. Food and Drug Administration (FDA) in its longstanding dispute with the agency regarding the approvability of HETLIOZ® (tasimelteon) to treat jet lag disorder (Vanda Pharmaceuticals Inc. v. FDA, case no. 24-1049).
Vanda submitted its supplemental New Drug Application (sNDA) in October 2018 to market HETLIOZ® to treat jet lag disorder. After the FDA substantially delayed resolving Vanda's request for a hearing with respect to this application, a federal district court found that the FDA "violated" the requirements of the Food, Drug, and Cosmetic Act, and the court ordered the FDA to finally resolve Vanda's sNDA or commence a hearing (Vanda Pharmaceuticals Inc. v. FDA, No. 22-cv-2775-CJN).
The FDA did not commence a hearing. It instead granted summary judgment to itself and issued an order refusing to approve the sNDA. The FDA took the position that it could essentially disregard the voluminous factual evidence Vanda had presented to it. Vanda thus filed a petition for review with the U.S. Court of Appeals for the D.C. Circuit (the Court).
In a sweeping win for Vanda, the Court set aside the FDA's action. The Court explained that Vanda provided expert views that were "specific, reasoned, and rooted in evidence" and that the FDA's "treatment of Vanda's evidence is cursory." In particular, the Court stated that Vanda "clearly offered meaningful evidence of tasimelteon's efficacy in improving sleep disturbance" and, further, that each of its trials "showed statistically significant improvement on the primary endpoint measured."
This decision significantly alters the relationship between the FDA and the parties it regulates. The Court's holding establishes that the FDA must meaningfully engage with the evidence presented by drug innovators, and the FDA may not shield its decisions via a plea for deference. The Court has remanded the case back to the FDA, where Vanda anticipates the FDA will either approve the sNDA or Vanda will receive a hearing.
This is an outstanding development for all those who suffer from jet lag. Vanda has spent more than a decade undertaking innovative clinical studies, now published in peer-reviewed journals, developing HETLIOZ® so that jet lag sufferers may finally have access to a meaningful therapeutic.1 2 3 4 Whether for an athlete, a business traveler, a government official, a tourist, or rapidly deployed troops, HETLIOZ® has the potential to fundamentally change the landscape of circadian resetting during transmeridian global travel. This is a treatment that Americans want and need.
Ultimately, this decision requires that the FDA act fairly when evaluating scientific evidence. The FDA's decisions regarding new drug applications are enormously consequential. Innovators like Vanda labor to bring novel therapeutics to Americans who suffer from unmet medical needs. Improper denials by the FDA deprive the public of access to potentially life-changing medication. The Court's decision establishes that the FDA must now actually engage with the evidence presented to it.
The Court's decision confirms Vanda's efforts to challenge unlawful actions by the FDA. For too long, drug manufacturers failed to exercise their rights to lawful treatment by the FDA. Vanda has demonstrated how courts can set aside illegal government actions that harm innovation and deprive Americans of important new therapeutics.
Vanda looks forward to further demonstrating that HETLIOZ® should be approved to treat jet lag disorder.
References
- Rajaratnam, S., Polymeropoulos, M., Fisher, D., Roth, T., Scott, C., Birznieks, G., Klerman, E. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet 2009; 373(9662):482-91. See also Cardinali, D., Golombek, D. Let there be sleep-on time. Ibid. 439-41.
- Polymeropoulos, C., Mohrman, M., Keefe, M., Brzezynski, J., Wang, J., Prokosch, L., Polymeropoulos, V., Xiao, C., Birznieks, G., Polymeropoulos, M. Efficacy of Tasimelteon (HETLIOZ®) in the Treatment of Jet Lag Disorder Evaluated in an 8-h Phase Advance Model; a Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Front Neurol. 2020; 11:611.
- Polymeropoulos, C., Polymeropoulos V., Cziesler, E., Fisher, M., Smieszek, S., Xiao, C., Birznieks, G., Polymeropoulos, M. Once-daily tasimelteon (VEC-162) for jet lag following transmeridian travel: A multicenter, randomized, double-blind, placebo-controlled trial. Front Neurol. 2022; 13:901467.
- See also Arendt, J. Approaches to the Pharmacological Management of Jet Lag. Drugs 2018;78(14):1419-1431.
About Vanda Pharmaceuticals Inc.
Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. For more on Vanda Pharmaceuticals Inc., please visit www.vandapharma.com and follow us on X @vandapharma.
About HETLIOZ®
For full U.S. Prescribing Information for HETLIOZ®, including indication and Important Safety Information, visit www.hetlioz.com.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements in this press release, including, but not limited to statements regarding Vanda's prospects for receiving approval of HETLIOZ® or a hearing following the FDA's further review of the sNDA following remand, the potential of HETLIOZ® to address the needs of travelers suffering from jet lag disorder and the anticipated future behavior of the judiciary, are "forward-looking statements" under the securities laws. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Forward-looking statements are based upon current expectations and assumptions that involve risks, changes in circumstances and uncertainties. Important factors that could cause actual results to differ materially from those reflected in Vanda's forward-looking statements include, among others, the outcome of the FDA's further review of the sNDA, the ability of HETLIOZ® to fundamentally change the landscape of circadian resetting during transmeridian global travel, and the willingness of other courts to set aside the actions of the FDA. Therefore, no assurance can be given that the results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Forward-looking statements in this press release should be evaluated together with the various risks and uncertainties that affect Vanda's business and market, particularly those identified in the "Cautionary Note Regarding Forward-Looking Statements", "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Vanda's most recent Annual Report on Form 10-K, as updated by Vanda's subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov.
All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this press release is provided only as of the date of this press release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Corporate Contact:
Kevin Moran
Senior Vice President, Chief Financial Officer and Treasurer
Vanda Pharmaceuticals Inc.
202-734-3400
[email protected]
Jim Golden / Jack Kelleher / Dan Moore
Collected Strategies
[email protected]
SOURCE Vanda Pharmaceuticals Inc.