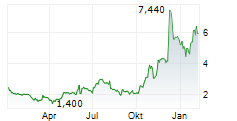
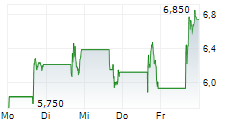
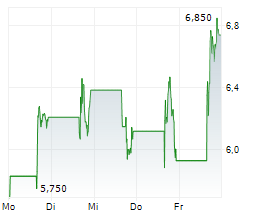
Immix has published its Q225 results, reflecting a productive period for NXC-201. The US-based NEXICART-2 trial in relapsed/refractory amyloid light chain amyloidosis (r/r ALA) reported an encouraging interim update and, given the current pace, Immix maintains its plans to file for regulatory approval once the trial concludes in mid-2026. Management also announced the potential expansion of NXC-201 into additional indications, which could increase the value proposition for the candidate. The company ended Q225 with net cash of $11.64m, which was bolstered post period by a further $1.34m through an at-the-market (ATM) offering. We estimate that this should provide a cash runway into Q126, but note the potential for this to extend should further proceeds be raised through the ATM facility. Following the Q225 results, our valuation for Immix stands at $126.8m, or $4.3 per share (from $127.0m, or $4.6/share previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research