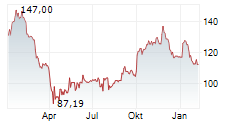
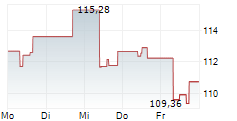
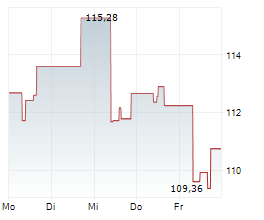
SANTA CLARA (dpa-AFX) - Agilent Technologies Inc. (A) Wednesday said that its MMR IHC Panel pharmDx, developed in partnership with Bristol Myers Squibb, has received approval from the U.S.Food and Drug Administration (FDA) as a companion diagnostic test for colorectal cancer.
This test helps to identify mismatch repair deficient colorectal cancer patients who are eligible for treatment with Bristol Myers Squibb's Opdivo alone or in combination with Yervoy.
'Our new CDx product offers healthcare providers an additional tool to identify mismatch repair deficiency in patients, complementing existing options and enhancing the ability to tailor immunotherapy treatments,' said Nina Green, vice-president and general manager of Agilent's Clinical Diagnostics Division.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News