CANBERA (dpa-AFX) - Telix Pharmaceuticals Limited (TLX, TLX.AX) announced that it has received a Complete Response Letter (CRL) from the U.S. FDA regarding its application to approve TLX250-CDx (Zircaix), a PET imaging agent used to help diagnose kidney cancer (specifically clear cell renal cell carcinoma).
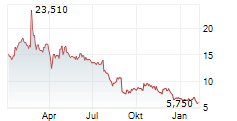
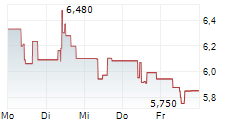
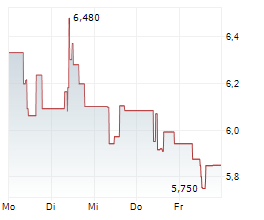
TLX closed wednesday regular tarding at $12.10 down $0.44 or 3.51%. The stock further dropped $2.20 or 18.18%.
The FDA found issues with the drug's manufacturing and quality control information. It has asked Telix to provide more data showing that the product used in clinical trials is similar to the one they plan to produce commercially. The FDA also flagged problems at two third-party manufacturing partners, which must be fixed before Telix can reapply.
Telix plans to meet with the FDA soon to discuss how to address these issues and when they can resubmit the application. Despite this setback, TLX250-CDx still holds Breakthrough Therapy and Priority Review status, showing its potential to meet an important medical need.
Importantly, this FDA feedback does not affect Telix's 2025 revenue forecast, since the company had not included sales from this product in its guidance. In the meantime, Telix will continue offering TLX250-CDx to patients through the FDA's expanded access program, pending further discussions.
For More Such Health News, visit rttnews.com
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News