ZEISS Medical Technology will showcase new ophthalmic innovations and market milestones at ESCRS from Sept. 12 - 15 in Copenhagen, Denmark:
- EFFICIENT, CONFIDENT DECISION MAKING: introducing AI-powered CIRRUS® PathFinder clinical support tool now CE mark approved; presenting a fully integrated diagnostic and SLT therapeutic solution for managing comorbid cataract/glaucoma patients.
- NEW STANDARDS IN SURGICAL EXCELLENCE: showcasing the DORC EVA NEXUS dual strategy surgical platform as part of ZEISS's expanding surgical workflow solutions.
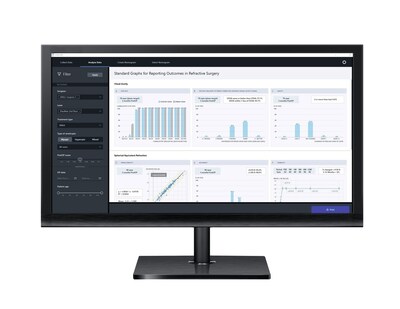
- IMPROVED PREDICTABILITY OUTCOMES: updated ZEISS VISULYZE 1.2 data analysis and personalized nomogram creation software tool now with ZEISS FORUM integration for more convenient and efficient nomogram creation within the ZEISS Corneal Refractive Workflow.
- REFRACTIVE INDUSTRY MILESTONES: more than 12 million eyes treated with ZEISS SMILE and ZEISS SMILE pro; also celebrating ongoing market traction of SMILE® pro for treating hyperopia.
- CATARACT MILESTONES: celebrating more than 10 million cataract surgeries using VisionBlue® anterior stain from DORC, a ZEISS company; celebrating key IOL milestones validated by multiple clinical studies presented at ESCRS.
JENA, Germany, Sept. 2, 2025 /PRNewswire/ -- ZEISS Medical Technology showcases the latest innovations and enhancements to its cataract and corneal refractive workflow solutions, from diagnostics and surgical planning to advanced treatments, at the European Society of Cataract and Refractive Surgeons (ESCRS) conference in Copenhagen, Denmark, from Sept. 12-15, 2025.
"ZEISS continues to introduce advanced optical and digital innovation to meet the expanding needs of Europe's leading refractive and cataract surgeons," says Magnus Reibenspiess, Head of Strategic Business Unit Ophthalmology at ZEISS Medical Technology. "From major industry milestones to new digital workflow solutions, our industry leadership shines bright, setting new standards for ophthalmic care."
Advancements deliver improved efficiencies in surgical planning
From advanced imaging to cloud-enabled data access, ZEISS diagnostic and digital solutions integrated into ophthalmic workflows can help surgeons visualize more, connect insights across a workflow, and make data-driven decisions, including earlier detection of eye conditions that may affect surgical planning and outcomes. At ESCRS, ZEISS will demonstrate a fully integrated solution connecting cataract and glaucoma workflows to support more efficient and confident decision making.
Glaucoma is the most common comorbidity in cataract surgery. According to a recent study1, around 20% of patients who undergo cataract procedures also have glaucoma or ocular hypertension. ZEISS offers integrated diagnostic and therapeutic solutions to support earlier detection and management of glaucoma in cataract patients, helping to improve long-term outcomes of cataract surgery. Devices including the ZEISS SL 800, CLARUS®, CIRRUS® OCT, ARTEVO® 750, and VISULAS® combi (yag/slt) are shared across the cataract and glaucoma workflows and seamlessly connected via FORUM® for complete data continuity.
"It's an exciting time to be an ophthalmologist. I'm extremely grateful to companies like ZEISS Medical Technology, that bring us products which are truly game changers in the care of our glaucoma and cataract patients," said Dr. Steven Vold, Founder and Chief Executive Officer of Vold Vision (Florida, USA).
ZEISS will also showcase CIRRUS® PathFinder, which recently received CE mark approval, an innovative clinical support tool with artificial intelligence (AI) fully integrated to enable more confident decision-making and accelerate a clinician's patient care workflow with OCT interpretation assistance, supporting improved surgical planning, particularly for high-volume clinics. The AI-powered OCT assessment tool is fully integrated into ZEISS CIRRUS2, using proprietary deep learning algorithms to automatically identify abnormal macular OCT B-scans as a licensed capability of the latest CIRRUS software release, helping to improve a practice's efficiency and patient care outcomes.
At ESCRS, the company will celebrate ZEISS CIRRUS PathFinder as a major step forward in AI and diagnostics, unveiling the progress made to date with the latest innovation in CIRRUS OCT technology including a presentation by Dr. Leo Seo Wei, MD, sharing her first impressions and expertise on Sunday, Sept. 14, at 3:00 PM CET in the ZEISS booth C1.130.
Leading the way in refractive surgery with SMILE® pro for hyperopia and advanced digitally connected patient planning
The ZEISS Corneal Refractive Workflow empowers surgeons to optimize patient flow while growing their practice with added value beyond the devices through targeted services that help improve patient outcomes through a digitally connected and streamlined clinical workflow. At ESCRS, ZEISS will showcase a new version of its comprehensive data analysis and personalized nomogram creation software tool, ZEISS VISULYZE 1.2, now connected to ZEISS FORUM so refractive surgeons can access their imported data with one click, offering convenience and efficiency during the nomogram creation process. The software update provides transparency into factors that can affect patient outcomes, allowing those insights to be transferred to the refractive laser as well as to the next treatment plan, reducing manual work and risks of transcription errors. With ZEISS FORUM integration, the ZEISS VISULYZE 1.2 software helps improve predictability and outcomes to support higher patient satisfaction.
ZEISS continues to extend its global refractive industry momentum with the celebration of more than 12 million eyes treated with ZEISS SMILE and ZEISS SMILE pro, reflecting the growing international adoption of the technology for a minimal invasive lenticule extraction procedure. The company is also celebrating one year of SMILE® pro for treating hyperopia, now available in 56 countries. ZEISS is the only provider offering a treatment for hyperopia with lenticule extraction, representing a promising advancement in refractive surgery with outcomes comparable to hyperopic LASIK. ZEISS SMILE and ZEISS SMILE pro continue to be leading solutions trusted by surgeons for the technology's proven reliability and effective treatment with the VisuMax® and VISUMAX® 800 from ZEISS.
ESCRS attendees can learn more about the latest refractive offerings within the ZEISS Corneal Refractive Workflow at the ZEISS booth C1.130, including a presentation on Sunday, Sept. 14, at 10:00 AM CET from Prof. Dr. Dan Reinstein, London Vision Clinic (UK), who will share his expert insights and valuable firsthand experience with ZEISS SMILE pro for hyperopia.
Celebrating industry milestones and unparalleled optics within an integrated cataract workflow
Setting new standards in surgical excellence, ZEISS is delivering industry-leading efficiency through digitally integrated solutions and unparalleled optics. At ESCRS, the ZEISS Cataract Workflow will include a robust portfolio of surgical solutions, including the 3D heads-up technology of the ZEISS ARTEVO 850, optical visualization with a novel light source with the ZEISS ARTEVO 750, the ZEISS EQ Workplace and ZEISS Surgery Planner digital cataract planning solutions, and the patented ZEISS QUATTRO pump of the QUATERA® 700 from ZEISS to experience chamber stability independent of IOP and flow. ZEISS will also showcase next to its primary cataract workflow the DORCEVA NEXUS platform, uniquely suited to address both retina and cataract surgery, now with availability of angled EquiPhaco designed for optimal irrigation flow. EquiPhaco, combined with EVA NEXUS SMART IOP, allows for a more stable, lower anterior chamber pressure during procedures.
ZEISS is also celebrating key product milestones, including a special event with Dr. Elena Barraquer in the ZEISS booth C1.130 on Saturday,Sept. 13, at 3:00 PM CET to highlight more than 10 million cataract surgeries conducted using VisionBlue® anterior stain from DORC, reflecting the solution's continued leadership in the market as the only trypan blue stain for anterior use approved by the FDA and now approved for staining of Descemet's membrane and trabecular meshwork.
Additionally, ZEISS is celebrating IOL clinical milestones which reflect the company's expertise in optics, featuring two distinct high-quality biomaterials and lens designs. Dr. Andrea Janeková from Eye Center Prague in Czech Republic says, "As the first peer-reviewed study on the AT ELANA® 841P, the data confirm that it delivers excellent distance, intermediate, and near vision with minimal photic phenomena, meeting the expectations of both surgeons and patients. What stood out most to me was the smooth and extended defocus profile of the AT ELANA 841P, which translated into functional vision across a wide range and high patient satisfaction." Dr. Mfazo Hove from Blue Fin Vision in the UK says, "I knew the AT LISA® tri lenses were good - my patients repeatedly told me. They have exceeded all my expectations since having them implanted 18 months ago. I might even have become a better surgeon - the numbers don't lie. PC rupture 0.2% off 5,600 operations in 12 months down from 0.27%."
As part of an IOL clinical milestone celebration in the ZEISS booth C1.130 on Saturday, Sept. 13, at 10:00 AM CET, the ZEISS IOL portfolio will be validated through multiple clinical studies and direct ZEISS IOL experience presented by industry experts including Prof. Gerd U. Auffarth (Heidelberg, Germany) and Dr. Kyungmin Koh (Seoul, Republic of Korea), as well as Dr. Andrea Janeková and Dr. Mfazo Hove.
ZEISS will showcase its latest offerings and new innovations at the European Society of Cataract and Refractive Surgeons (ESCRS) conference from Sept. 12 - 15, 2025, at booth C1.130.
For more information, visit www.zeiss.com/med.?
1 Skalicky, S.E., Martin, K.R., Fenwick, E., Crowston, J.G., Goldberg, I. and McCluskey, P. (2015), Cataract and quality of life in glaucoma. Clin Experiment Ophthalmol, 43: 335-341. https://doi.org/10.1111/ceo.12454.
2 ZEISS PathFinder works on all current ZEISS CIRRUS devices: 500, 5000, 6000, however RDB2 is for ZEISS CIRRUS 6000 data only.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates to not have clinical evidence supporting the opinions and statements of the health care professionals nor accept any responsibility or liability of the healthcare professionals' content. The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support.
Contact for investors
Sebastian Frericks
Head of Group Finance & Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220 116
Mail: investors.med@zeiss.com
Contact for the press
Frank Smith
Head of Global Communications Ophthalmology
Carl Zeiss Meditec Inc.
Phone: +49 3641 220 331
Mail: press.med@zeiss.com
www.zeiss.com/newsroom
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With 5,730 employees worldwide, the Group generated revenue of €2,066.1m in fiscal year 2023/24 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 39 percent of Carl Zeiss Meditec AG's shares are in free float. Approx. 59 percent are held by Carl Zeiss AG, one of the world's leading groups in the optical and optoelectronic industries.
For further information visit: www.zeiss.com/med
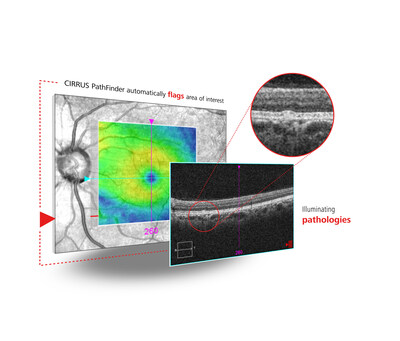

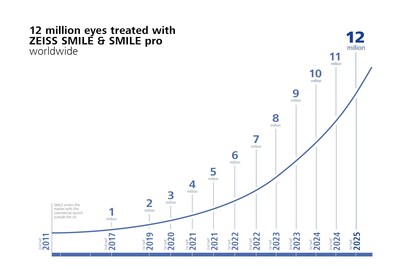


Photo - https://mma.prnewswire.com/media/2762265/VISULYZE_Monitor.jpg
Photo - https://mma.prnewswire.com/media/2762266/ZEISS_CIRRUS_PathFinder.jpg
Photo - https://mma.prnewswire.com/media/2762267/EVA_Nexus.jpg
Photo - https://mma.prnewswire.com/media/2762268/SMILE_campaign_graphic.jpg
Photo - https://mma.prnewswire.com/media/2762269/VBL_10_million.jpg
Logo - https://mma.prnewswire.com/media/546786/ZEISS_v1_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/zeiss-showcases-expansion-of-ophthalmic-care-options-creating-industry-leading-workflow-solutions-marks-new-refractive-and-cataract-milestones-302543589.html
View original content:https://www.prnewswire.co.uk/news-releases/zeiss-showcases-expansion-of-ophthalmic-care-options-creating-industry-leading-workflow-solutions-marks-new-refractive-and-cataract-milestones-302543589.html