BRUSSELS (dpa-AFX) - UCB SA (UCBJY), Wednesday announced has released three-year results from its BE HEARD trials demonstrating that BIMZELX or bimekizumab-bkzx, the first and only approved therapy targeting both IL-17A and IL-17F, provided sustained symptom relief and was generally well tolerated in adults with moderate-to-severe hidradenitis suppurativa.
The findings showed that improvements achieved after one year were maintained through three years, with more than 90 percent of patients sustaining HiSCR50 responses and half achieving complete resolution of nodules and abscesses.
Quality of life measures also improved, with 38 percent of patients reporting no impact from HS after three years. Outcomes were particularly strong in patients treated earlier after diagnosis.
Long-term safety remained consistent with earlier data, with no new signals observed. The results, presented at the 2025 European Academy of Dermatology and Venereology Congress in Paris, reinforce BIMZELX's potential as a durable treatment option for HS.
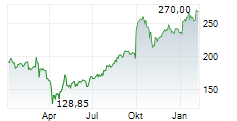
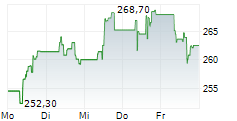
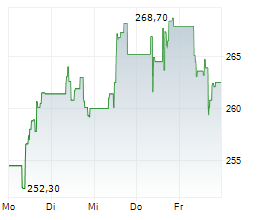
UCBJY is currently trading at $120.98, down $0.18 or 0.15 percent on the OTC Markets.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News