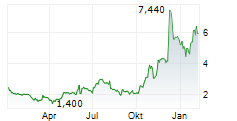
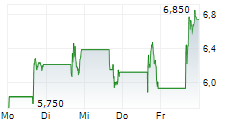
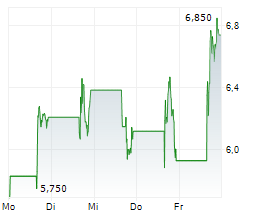
Immix Biopharma has announced that its US-based NEXICART-2 trial, evaluating lead CAR-T asset NXC-201 in amyloid light chain amyloidosis (ALA), has surpassed the 50% enrolment milestone. The news provides affirmation that this key study is progressing to plan, in line with prior guided timelines. Enrolment will continue to be a strategic priority as management works toward a biologics licence application (BLA) submission, which we estimate will take place after the trial concludes in mid-2026. Should the clinical data continue to be supportive, NXC-201 could become the first CAR-T therapy for ALA, a debilitating condition that currently lacks durable treatment options.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research