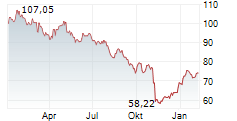
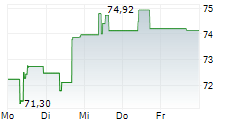
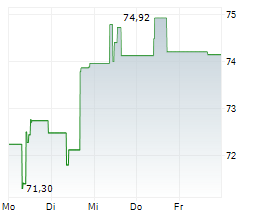
ROME (dpa-AFX) - DiaSorin S.p.A. (DIA.MI) on Friday announced the launch of its new LIAISON TSH-R Ab assay, now available in all countries recognizing the CE Mark.
This test is designed to help diagnose and monitor patients with suspected Graves' Disease, an autoimmune thyroid disorder.
The new LIAISON TSH-R Ab test improves the management of patients with Graves' Disease by allowing effective monitoring of treatment. It uses a new double-bridge sandwich assay technology, which greatly enhances accuracy and reliability.
The new test adds to Diasorin's comprehensive LIAISON Thyroid and Autoimmune Thyroid panels, offering laboratories a complete thyroid testing solution on one platform.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News