Regulatory News:
TME Pharma N.V. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company specializing in the development of novel therapies for cancer and eye diseases, announces today that its half year results will be published on 30 October 2025. The closed period for insiders of the Company will start next week, 30 days before the publication of the half-year results on October 30, 2025.
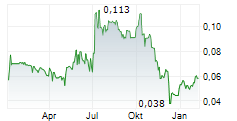
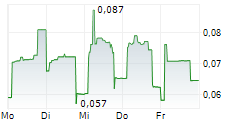
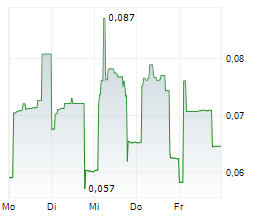
TME Pharma additionally announces that it has received notification that Diede Van Den Ouden, Chief Executive Officer and a Person Discharging Managerial Responsibilities ("PDMR"), has purchased an additional 300,000 ordinary shares in the Company at an average price per share of €0.0927. The shares were acquired through an on-market transaction on Euronext Growth Paris on Friday 19 September 2025. Following this transaction, Mr Van Ouden holds a total of 2,300,000 ordinary shares.
Statement from the CEO
"My share purchases are an indication of my strong belief in the Company's future, financial position and ability to progress its research and development activities. I expressly reserve the right, when permitted and under appropriate circumstances, to purchase shares in the Company in the market. I am fully aligned with fellow shareholders, having elected only a modest cash remuneration, because the true success of this project rests on the future value of my equity." said Van den Ouden, CEO
"Although we have not yet disclosed concrete news, I am pleased with the progress made during the first three months of my tenure including the aggressive cost-saving measures, which have reduced monthly operating expenses to approximately €100,000 per month, enabling the Company to report a cash balance of €2.35 million at September 1, 2025. The transition to a virtual model has been smooth-the former team remains available to support us and continues to be motivated to succeed. We are actively engaging in discussions with a broad spectrum of potential partners relating to the Company's research and development programs and other assets. At the same time, we have reaffirmed our commitment to profitability outside of biotech investments by pursuing the right partnerships, acquisitions, and ventures and other revenue generating initiatives."
About TME Pharma
TME Pharma is a clinical-stage biotechnology company specializing in the development of novel therapies for cancer and eye diseases. The Company's lead compounds have been designed to act on the tumor microenvironment (TME) and the cancer immunity cycle by breaking tumor protection barriers against the immune system and blocking tumor repair. The Company's two lead assets are:
- NOX-A12 (olaptesed pegol, an anti-CXCL12 L-RNA aptamer), which is being studied (GLORIA Phase 1/2 clinical trial) in newly-diagnosed brain cancer patients who will not benefit clinically from standard chemotherapy. The US FDA and the German BfArM have approved the design of a randomized Phase 2 trial in glioblastoma, and TME Pharma was awarded Fast Track Designation by the FDA for NOX-A12 in combination with radiotherapy and bevacizumab for use in the treatment of the aggressive adult brain cancer, glioblastoma. NOX-A12 in combination with radiotherapy had also previously received orphan drug designation (ODD) for glioblastoma in the United States and glioma in Europe.
- NOX-E36 (emapticap pegol, L-RNA aptamer inhibiting CCL2 and related chemokines), which is being evaluated in ophthalmic diseases with a high need for well-tolerated therapies with anti-fibrotic effect.
The Company, under the leadership of its new CEO, Diede van den Ouden, who joined in the June 2025, is currently undertaking a strategic restructuring with the goal of providing the financial resources to unlock the value of NOX-A12 and NOX-E36. These steps include:
- Raising funds from alternative sources (€1.7 million raised in May 2025, including €500,000 from the new CEO)
- Pursuing stable, cash-generating business opportunities to achieve positive operational cash flow for the Company
- Leveraging tax loss carry forwards
- Gaining exposure to digital assets via newly established crypto brokerage account
Further information can be found at: www.tmepharma.com.
About the GLORIA Study
GLORIA (NCT04121455) is TME Pharma's dose-escalation, Phase 1/2 study of NOX-A12 in combination with radiotherapy in first-line partially resected or unresected glioblastoma (brain cancer) patients with unmethylated MGMT promoter (resistant to standard chemotherapy). GLORIA further evaluates safety and efficacy of NOX-A12 in the expansion arm in which NOX-A12 is combined with radiotherapy and bevacizumab.
About the OPTIMUS Study
OPTIMUS (NCT04901741) is TME Pharma's planned open-label two-arm Phase 2 study of NOX-A12 combined with pembrolizumab and nanoliposomal irinotecan/5-FU/leucovorin or gemcitabine/nab-paclitaxel in microsatellite-stable metastatic pancreatic cancer patients.
Disclaimer
Translations of any press release into languages other than English are intended solely as a convenience to the non-English-reading audience. The company has attempted to provide an accurate translation of the original text in English, but due to the nuances in translating into another language, slight differences may exist. This press release includes certain disclosures that contain "forward-looking statements." Forward-looking statements are based on TME Pharma's current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Factors that could cause actual results to differ include, but are not limited to, the risks inherent in oncology drug development, including clinical trials and the timing of and TME Pharma's ability to obtain regulatory approvals for NOX-A12 as well as any other drug candidates. Forward-looking statements contained in this announcement are made as of this date, and TME Pharma undertakes no duty to update such information except as required under applicable law.
Management will now iParallel to its core biotechnology activities, the company is exploring potential acquisitions and partnerships in stable, profitable businesses. These efforts are aimed at creating a fundamentally profitable corporate structure in which revenues from non-core activities will support and strengthen the further development of its patented drug candidates, which remain the company's flagship products, NOXA12 and NOX-E36.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250921266307/en/
Contacts:
For more information, please contact:
TME Pharma N.V.
Diede van den Ouden, CEO
ir@tmepharma.com