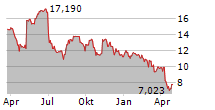
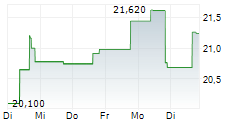
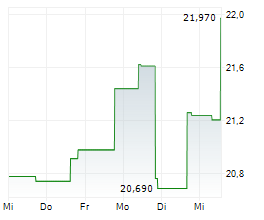
WASHINGTON (dpa-AFX) - Elanco Animal Health Incorporated (ELAN) on Tuesday said that the U.S. Food and Drug Administration (FDA) has approved an updated label for its Zenrelia, removing language related to vaccine-induced disease.
Zenrelia, approved by the FDA in September last year, is indicated for controlling itching and inflammation caused by skin allergies in dogs aged 12 months and older. At launch, the U.S. Zenrelia label included warnings based on a laboratory study where unvaccinated dogs were given three times the recommended dose. An outbreak of other illnesses during the study made the results hard to interpret.
After reviewing additional data, the FDA concluded that the overall evidence supports removing the warning about fatal vaccine-induced disease from modified live virus vaccines. This language has now been removed from the U.S. Zenrelia label.
However, the label's Boxed Warning continues to advise discontinuing Zenrelia at least 28 days to 3 months prior to vaccination and withholding it for at least 28 days after vaccination, due to the risk of a reduced immune response.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News