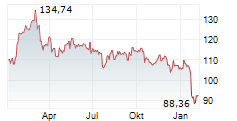
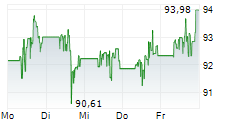
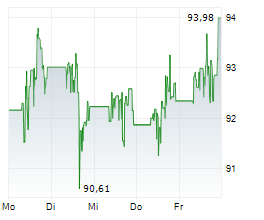
NORTH CHICAGO (dpa-AFX) - Abbott Laboratories (ABT) on Monday announced that Health Canada has approved its dissolvable stent, the Esprit BTK Everolimus Eluting Resorbable Scaffold System.
The device is used to treat people with chronic limb threatening ischemia (CLTI) below-the-knee (BTK). It keeps arteries open and delivers the drug everolimus to support healing before the stent fully dissolves.
More than 800,000 Canadians are living with Peripheral Artery Disease (PAD). CLTI is a severe form of PAD in which arteries become clogged with plaque, blocking blood flow and oxygen to the lower leg and foot. People with CLTI often experience severe pain and non-healing open wounds.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News