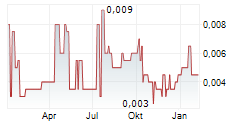
Percheron Therapeutics has reported final results from the Phase I dose escalation study of HMBD-002, its monoclonal antibody targeting VISTA, a novel immune checkpoint protein. The data confirmed the compound's favourable safety and tolerability profile (maximum tolerated dose not reached with <10% of patients experiencing grade 3 or greater adverse events), with early signs of disease control in advanced solid tumours. The trial was not designed or sufficiently powered to demonstrate efficacy; however, evidence of stable disease (28% of cases) in an otherwise heavily pre-treated patient population (median four to five prior lines of treatment) supports Percheron's move towards Phase II development in CY26. We expect the announcement on the Phase II design and target indications, due in Q4 CY25, as the next big catalyst for the company.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research