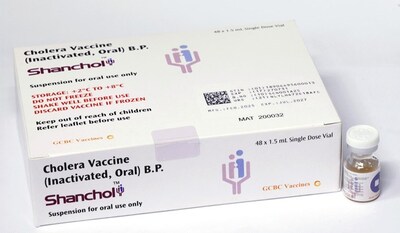
HYDERABAD, India, Oct. 8, 2025 /PRNewswire/ -- Shanchol®, the oral cholera vaccine originally developed by Shantha Biotechnics, has received prequalification from the World Health Organization (WHO). This milestone enables global procurement agencies such as UNICEF, Gavi, and PAHO to source Shanchol® for use in countries where cholera remains a major public health challenge.
"Shanchol® was conceived as an affordable, accessible solution for countries facing repeated cholera outbreaks. The WHO prequalification of Shanchol carries forward the founding mission," said Dr. K.I. Varaprasad Reddy, Founder of Shantha Biotechnics.
Over the years, close to 40 million doses of Shanchol® have been supplied worldwide through UNICEF-led vaccination campaigns. After production was paused under its former ownership, GCBC Vaccines Pvt. Ltd. (formerly Shantha) has resumed manufacturing, with WHO conducting an on-site inspection and transferring the prequalification. Shanchol® remains the only oral cholera vaccine manufactured in India with WHO prequalification, underscoring India's critical role in ensuring continuity of global supply.
"As part of the founding family of Gland Pharma, I have seen how world-class sterile manufacturing from India can transform global healthcare. By resuming production of Shanchol®, we are bringing the same rigor and reliability to ensure this life-saving vaccine remains available worldwide," said Dr. Ravi Penmetsa, Managing Director, GCBC Vaccines Pvt. Ltd.
"This milestone underscores Shantha's renewed commitment to global health. Our focus is on ensuring that vaccines like Shanchol® reach the countries that need them most, reliably and affordably. At the same time, we are working to bring other affordable and innovative vaccines from our pipeline to global markets, continuing Shantha's tradition of expanding access to life-saving immunization," said Dr. Vishy Chebrol, Executive Director, GCBC Vaccines Pvt. Ltd.
About Shanchol®
Shanchol® is a bivalent killed whole-cell oral cholera vaccine, effective against Vibrio cholerae O1 and O139. It has been a cornerstone of UNICEF-coordinated outbreak responses and preventive immunization campaigns, protecting millions of people worldwide. With WHO PQ approval, Shanchol® will continue to be supplied at scale to meet international demand and country-level immunization needs.
About the Manufacturer
Shanchol® was originally developed by Shantha Biotechnics, founded in the 1990s as one of India's pioneering biotech firms. The company was acquired by Sanofi in 2009, gaining global recognition as a trusted vaccine supplier. In 2024, Shantha's facilities including Shanchol®, were acquired by GCBC Vaccines Pvt. Ltd. Today Shantha's legacy is continued, through the revival of the company and its mission to deliver high-quality, affordable vaccines that expand access to life-saving immunization worldwide.
For information, visit https://gcbcvaccines.com/
Photo: https://mma.prnewswire.com/media/2791441/Shantha_GCBC_Shanchol_WHO_PQ.jpg
Logo: https://mma.prnewswire.com/media/2791442/Shantha_GCBC_Vaccines_Logo.jpg

![]() View original content:https://www.prnewswire.co.uk/news-releases/who-prequalifies-shanchol-oral-cholera-vaccine-ensuring-global-supply-continuity-302578406.html
View original content:https://www.prnewswire.co.uk/news-releases/who-prequalifies-shanchol-oral-cholera-vaccine-ensuring-global-supply-continuity-302578406.html